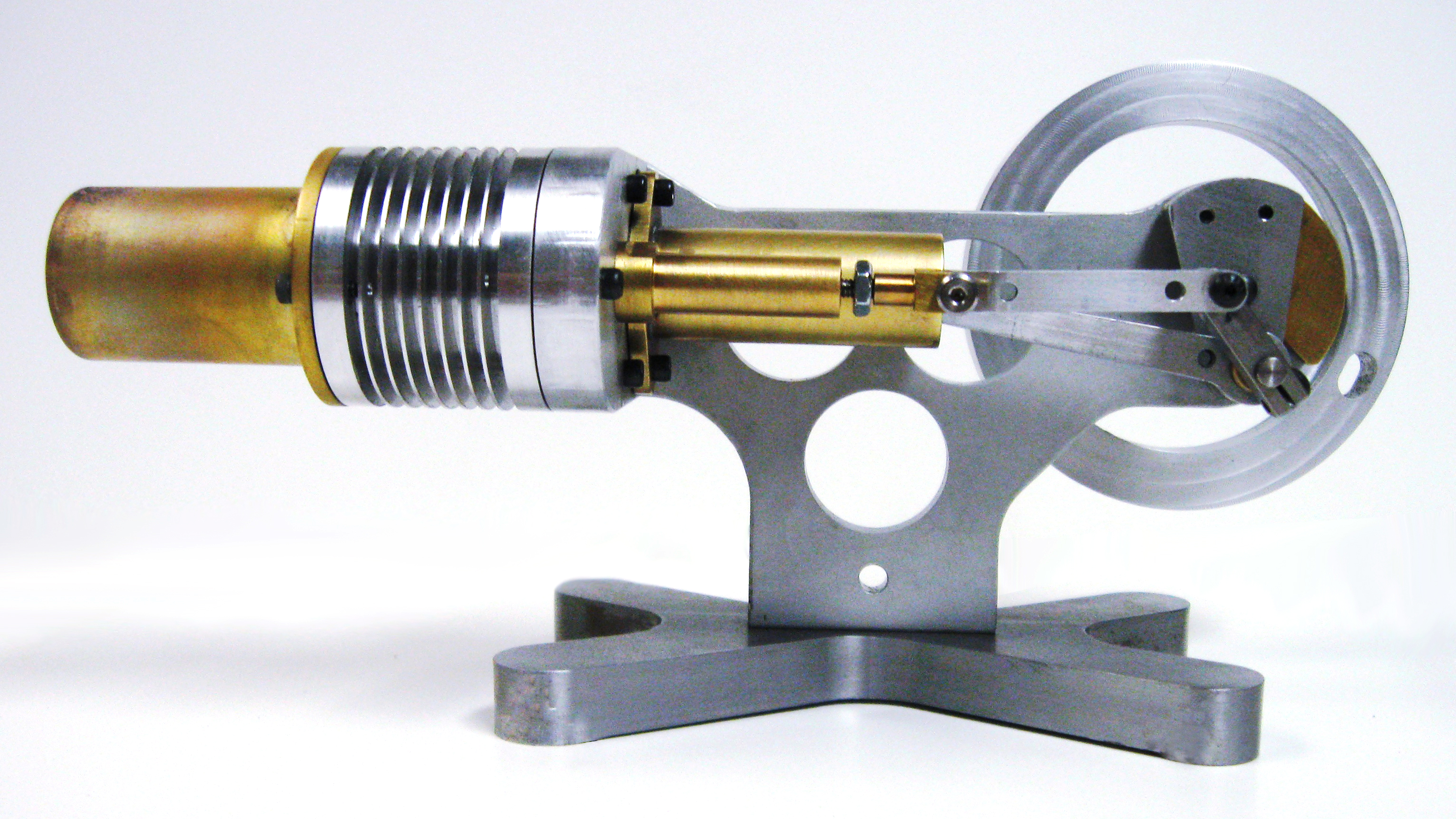
Stirling Engines
| Equation: |
Description: |
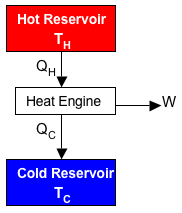
| Conservation of Energy Principle |
|
| For a cycle, the initial and final state
of the system are the same and thus have the same amount
of energy. |
|
| Because there is no change in energy
between the initial and final states of the cycle, all
added energy must leave within the same cycle. |
|
| The only energy added to the system in
heat from the Hot Reservoir. |
|
| Energy leaves the cycle at the cold
reservoir and in the form of work. (Ideally only in
those two forms, in reality it would include far more
forms). |
|
| Rearranging the energy balance, these are
all the ways in which energy is transfer to or from a
Stirling Engine. |
|
| Solving the energy balance for work
output, in terms of heat transfer in and out. |
|
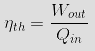 |
Another bit of information one might be
interested in is the thermal efficiency (ηth)
of the Stirling
Engine. |
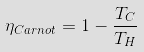 |
Theoretically, the highest efficiency for
a heat engine is the Carnot Efficiency, which can be
calculated if one knows the absolute temperatures of the
hot and cold reservoirs |
From the above, one can, knowing a few values determine the
work a Stirling engine can produce, how efficient it is and
compare its efficiency to the maximum efficiency that a heat
engine can have given the temperature of its' reservoirs.
The math above applies to all Stirling Engines, so if you'd
like to learn about the variations, click here.
Professor: David Newman, Ph.D.
Course: PHYS 212
Semester: Spring 2017
Student: Riley Bickford