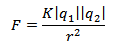
(Formula done in Microsoft Word)
where K is the electrostatic
constant, r is the distance between the two particles
(electron and nucleus, in this case), and the two q's
are the charges of the particles. This formula is very
basic, and only deals with particles as points
(spherical cow physics at it's finest). In order to get
the electrons to break free from their orbits around the
nucleus of an atom, an outside force must act upon the
system to effectively "break" the attractive force that
already exists. To overcome this bond, there needs to be
a steady supply of energy, whether it's electric,
electromagnetic, or thermal, to heat things up. If the
supply of energy is cut off or is insufficient, then the
electrons and positive ions can reform into atoms and
molecules once again. In general, gases turn into plasma
when the temperature is around 1000 Kelvins, or 726.85
degrees Celsius. Professor William Howard from the
University of Alaska, Fairbanks notes that plasma can
also be made by heating an ionic solid, but for our
purposes, we will be focusing on plasmas that are made
from a gaseous state. Here's a neat diagram showing some
different plasmas and the temperatures they form at:
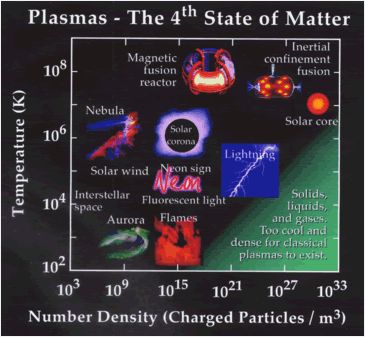
(I guess you could say it's a hot topic? Sorry, that was too easy)
(www.plasmas.org/what-are-plasmas.htm)

(I guess you could say it's a hot topic? Sorry, that was too easy)
(www.plasmas.org/what-are-plasmas.htm)
We now know what plasma is and
how it's formed. Let's get to the fun stuff, then:
taking a look at different plasmas that we can see
right here, right now!