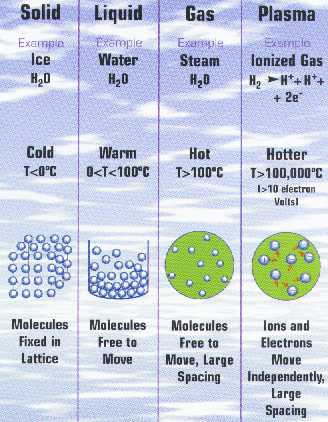
(www.plasmas.org/what-are-plasmas.htm)
Plasma is special in that it
exhibits properties of gas, such as not having shape
or volume unless confined in a container and having
very large distances between particles, but is also an
excellent conductor because of all those free
electrons (unlike gases; gases are notoriously poor
conductors), and also can be directed by a magnetic field.
However, the question is, how did those electrons
become so footloose and fancy free? Aren't electrons
and the nucleus of an atom bonded together tightly?
The answer lies in the next page.