|
Step 1: Fuel Source
Gas grills
typically use propane gas, an energy-dense fossil
fuel. Propane itself is not actively searched for
and produced, but rather it is a by-product of
distilling natural gas and refining petroleum.
Natural gas and petroleum are formed by the organic
material of dead plants and animals. Millions of
years ago this matter accumulated at the bottom of
oceans and other bodies of water, and were
subsequently covered by layer upon layer of sediment
due to earths natural processes. Subjected to high
pressures and temperatures deep beneath the earths
surface, the organic material is broken down and
forms either natural gas or petroleum based on the
amount of hydrogen and carbon present. 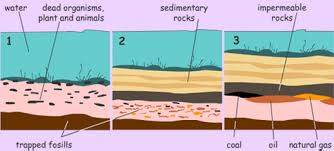
 
http://teambiofuels.weebly.com/fossil-fuels.html Part 1 -----> https://www2.dteenergy.com Part 2 -----> http://www.homedepot.com Part 3 Surprisingly, the propane tank connected to your gas grill contains very little gas! Instead, it contains liquid propane, abbreviated LP. Propane is an easily compressible gas due to its chemical structure, and we have taken advantage of this property. Propane has a boiling point of around -42 C. This means that unless you live in Fairbanks, Alaska or some other Nordic region with god-awful winter temperatures, your propane will almost always be in gas form. But due to the expansive nature of gases, storing propane in gas form at normal temperatures would be very inefficient for daily grilling use. However, with some rearranging of the ideal gas law: PV=nRt ----> P=nRt/V
|
Step 2: Transportation of Fuel to Stable Combustion Environment
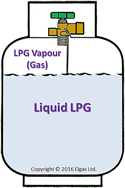
| When you turn
the propane tank "on" what you are actually doing is
opening a small whole which allows the propane in
the tank to escape through the gas line of the grill
to the burners. Even though the vast majority of
moles of propane in the tank are in liquid form,
there is still some vapor in the tank. The propane
vapor is located at the top of the tank due to
having a lower density than its liquid form. The
vapor is what pushes its way into the opening and
travels through the gas line. As the propane vapor
leaves the tank, one might think that the pressure
will decrease. However, this is not the case. Once a
certain amount of moles of the gas leaves, the same
moles of propane boil into the vapor phase and
replaces the pressure that should be "lost". Thus,
we can say that the tanks pressure is in equilibrium.
This remains the case until all the liquid is
used up, at which point the pressure will start to
decrease as gas escapes. |
 https://qph.ec.quoracdn.net/main-qimg-3168b87efc17867c3cd76be014898005 |
Step 3: Conversion of Propane to Heat
| Now that we
understand the propane used in our grill, we can
feel comfortable using it to grill our steak.
However, we still lack some understanding of the
process, namely how is the propane used to
produce heat? Once the propane reaches the burner, a predictable process takes place: the burning of propane gas. The propane flows evenly through the burner holes and a small spark is added through the "starter" switch of the grill which in turn ignites all the propane and produces the flames you see. This is an example of a combustion reaction. A combustion reaction is a chemical reaction in which a substance and oxygen are combined to produce heat, light, and the by products of the reaction. The heat and the light components are evident, however the reaction is also producing carbon dioxide and water. The balanced combustion reaction of propane is shown below. C3H8 + 5
O2 → 3 CO2 + 4 H2O + heat
We now have turned our
propane into heat, and we are only a preheat and
4 minutes on each side away from eating!
|
Title Page Method 1: Gas Grill Method 2:Charcoal Grill Understanding The Cooking Process Bibliography