| The key concept
to cooking our steak is heat. Heat itself is
not a form of energy, rather it is a transfer of
energy. The heat produced by our fuel sources is what
cooks our steak, and the physics involved is both
beautiful and appetizing. |
Preheat
When we use
our grill, the first step is to preheat it to a
constant temperature. When our fuel undergoes
combustion, heat is released into our enclosed
grill. However, the heat doesn't simply stay
confined. The second law of thermodynamics states
that heat is always transferred from a hotter to a
colder system. As heat buildups in our enclosed
grill, it also being released to the cooler,
surrounding environment. The heat escapes through
leaks in the seal of our enclosed grill, designed
holes in the grill itself, and by the surface of the
grill enclosure which conducts the heat which is
then released to the environment. The hotter our
grill gets, the heat escapes due to the temperature
difference between the the enclosure and the
environment. Eventually, our grill will not get any
hotter and it has reached an equilibrium, where the
heat loss to the environment is equal to the heat
gain by our fuel source. 
 http://www.thedailymeal.com/cook/how-make-restaurant-quality-burgers-your-backyard-barbecue-slideshow/slide-8 http://dc-cdn.s3-ap-southeast-1.amazonaws.com/dc-Cover-rkk7kibkdqvknsqp4qbv2tsq01-20160315015750.Medi.jpeg |
Grilling
| As our steak is presumably colder than our grill, heat will be transferred to it once we put it on. There are three different heat transfer processes, and all are being used to cook our steak. |
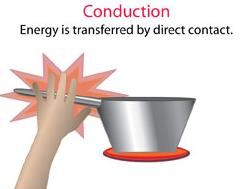
| Conduction Conduction is the heat transfer due to objects being in contact with one another. While this seems straight forward, an analysis at the particle level is quite interesting. The "hot reservoir" (which in this case is our grill grates) contain particles with a higher kinetic energy than the "cold reservoir" (the raw meat). When we put our steak on the grill, these faster mover particles collide with the slower moving particles of our steak, transferring kinetic energy. This energy speeds up the steak particles, which is why it gets warmer. Thus the heat transfer is simply the transfer of energy from physical collisions between our hot and cold reservoirs. |
 http://mechanicalbuzz.com/wp-content/uploads/2016/03/conduction.jpg |
 |
Convection Convection is the transfer of heat by the movement of fluids, such as air or water. The majority of the energy that cooks the steak is due to convection heating. As our grill reaches its preheated temperature, the air inside of it is circulating around and carries thermal energy with it. This fluid of hot air surrounds our steak and transfers energy in a similar manner as conduction heating. |
| Radiation Radiation is the heat transfer that occurs due to electromagnetic wave propagations. Unlike the physical collisions between molecules which transfer energy in convection and conduction, the electromagnetic waves excite the steak particles and cause them to speedup without an interaction of matter. The radiation emitted is due to the electromagnetic waves from the grills flames. The flames we see are really just heated up electrons, which emit electromagnetic waves in the form of light and energy as they drop back to a lower energy level. This EM wave emission propagates through our steak, transferring energy which helps to cook it. |
 |
"How Would You Like That Cooked?
 |