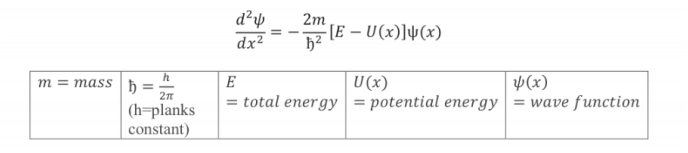
Schrodinger Equation

Newton produced the laws of classical physics, and Erwin Schrödinger produced the law of Quantum Mechanics. Schrödinger’s equation allows us to produce a wave function which is used to asses matter and “predict the probability of detecting it in some region. (text)” Though like de Broglie’s wavelength models, which accounted for the Hydrogen atom, Schrödinger’s quantum mechanics can accurately describe all the atoms of all the elements!