Dalton's
Law
Dalton's law states that the total pressure
exerted by a gas is equal to the sum of the pressures exerted by each
gas in the mixture. The part of the total pressure exerted by each gas
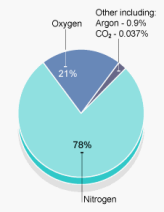
is referred to as the partial pressure. In air, the total pressure is
due to the partial pressure of nitrogen, plus the partial pressure of
oxygen, plus the partial pressure of each of the trace gases.
PTotal
= P1 + P2 + P3 +...
What Dalton discovered is that all gases compress equally and therefore
the ratio of gases remains
the same as the pressure increases. As the diver descends
deeper, more pressure is put on the breathing gas, compressing it in
the hose of the regulator, yet the volume of the divers breath is
remaining roughly the same. Therefore, as a diver descends, he or she
is breathing higher concentrations
of both oxygen and nitrogen than would be experienced at the surface.
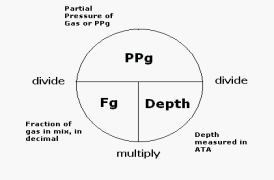
The table to the right is found in many introductory SCUBA
manual and may seem confusing, but remember, the partial pressure of a
gas can be found by taking the fraction of a gas in the mixture, in
decimal form (percentage/100), times the depth in atmospheres absolute,
where atmospheres absolute is the number of feet on you depth gauge
divided by 33, plus one. This calculates the number of atmospheres a
diver is experiencing from the water they are submersed in and add an
atmosphere of pressure to account for the pressure of the earth's
atmosphere.
|
Partial pressure formula
Scuba Tech Philippines
partial
pressure
=
fraction of gas in mix
×
atm
absolute
atm absolute =
(depth (ft) / 33) + 1
|