Science Photo Library
Another interesting law of
physics
divers must be aware of is Charle's law. Charle's law states that if the
volume of a gas in a container remains constant, but the temperature
increases, the pressure of the gas increases. The converse is also
true. if the temperature of a gas decreases, the volume of the gas
decreases.
Scuba Tech Philippines
This explains why if your warm tank reads less pressure
after you jump
into the cool water with it. The gas is cooling and
contracting and therefore you have less pressure in your tank.
|
To
begin
to understand the
importance that gas laws hold to a SCUBA diver, one must realize that
at the surface of the water, the diver is feeling one atmosphere of
pressure. As the diver descends, however, pressure is put on him or her
due to the weight of the water above. At a depth of about 10 meters in
seawater, the diver is experiencing around 2 atmospheres of pressure.
This is twice the pressure at the surface.
This pressure continues as the diver descends.
Imagine you have a
closed balloon full of air. As the balloon descends in the water
column, it will shrink! This is because greater pressure is being
placed on the gas and compressing it. This relation is given by Boyle's Law which states that
absolute pressure applied to a gas is inversely proportional to the
volume of that gas. In other words, as the pressure on the gas
increases, the volume of the gas decreases. This is given by the
following equation:
P=1/V
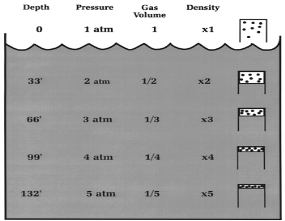 Boyle's Law
Boyle's Law
Scuba Tech Philippines
As a diver, Boyle's law tells
you that as you descend, gases in your body and equipment decrease in
volume. If a diver descends and does not exhale any air through his or
her nose, he or she may experience what is called a mask squeeze. This
is when the small volume of air in the divers mask decreases with depth
and causes the mask to suction to the divers face. In some cases the
suction is so powerful that it can break capillaries in the eyes and
face.
 Mask squeezes can be painful
Mask squeezes can be painful
aquaview.net
As a diver ascends, the
pressure they experience decreases and therefore the volume of gases in
their body and equipment increases. By this same law, the air in a
diver's lungs increases. If this air is not allowed to escape the
lungs, the small air-holding sacks, or alveoli in the lungs can burst.
Therefore, the number one rule in SCUBA diving is:
Never hold your breath!
|

