Thermodynamics
Entropy - The disorder of matter. *Entropies of more complex molecules are larger then those of simpler molecules, especially in a series of closely related compounds. (2)
Second Law of Thermodynamics - The total entropy of the universe is continually increasing. (2)
Protein stability depends basically in the free energy change between the folded and unfolded states, which is expressed by,
Where R represents the Avogadro number, K, the equilibrium constant, G, the free energy change between folded and unfolded, H, the enthalpy change and S, the entropy change from folded to unfolded. The enthalpy change, H, corresponds to the binding energy (dispersion forces, electrostatic interactions, van der Waals potentials and hydrogen bonding) while hydrophobic interactions are described by the entropy term, S. Proteins become more stable with increasing negative values of, G. As the binding energy increases or the entropy difference between the two states decreases, the folded protein becomes more stable. (4)
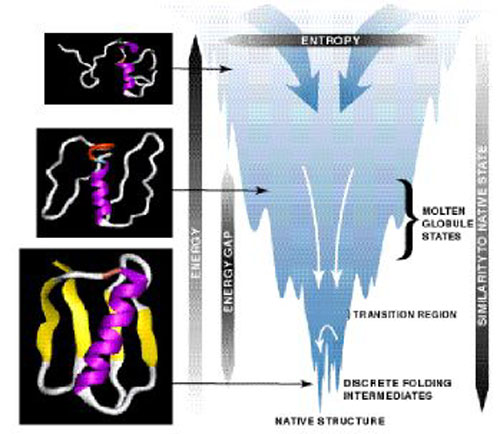
The thermodynamics of protein stability can best be model by the Energy Landscape Theory. This describes where the energy of a protein is a function of the topological arrangement of the atoms. A spatial surface with a very large number of different co-ordinates and energy values separated by mountains and ridges. Each value in this surface describes the protein in a specific conformation, and there is an energy landscape for each state of the protein. (4)

Figure 4. The Energy Landscape Theory Model