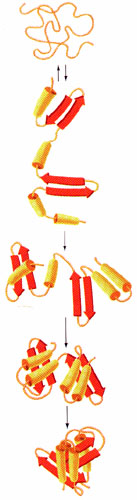
Figure 3. Folding Pathway(5)
Protein Stability
As odd as it may seem native (folded) proteins are only marginally stable under physiological conditions. (5) Other forces such as hydrophobic effects, electrostatic interactions, and hydrogen bonding act more as stabilizing factors and are the main factors in driving the protein folding process. (4)
- Hydrophobic effects cause the nonpolar substances to minimize their contact with water, which is the major determinant of native protein structure. The aggregation of the nonpolar side chains in the interior of a protein is favored by the increase in entropy of the water molecules that would otherwise form ordered cages around the hydrophobic groups.(5 )
- In the interior of the protein where the molecules are closely packed, van der Waals forces are relatively weak, but only act for a short time because these forces are lost when the protein is unfolded.
- Hydrogen bonding is important because proteins fold in such a way to prevent hydrogen bonds, because the stabilizing energy of the hydrogen bond would be lost when folding and unfolding occurred.
Largely the residues that occupy the interior of the protein direct protein folding. Returning to its native conformation occurs within a few seconds, which supports the idea that proteins have direct pathways to arrive at the native state. As the protein folds its free energy decrease which makes it a one-way process. The last stages of protein folding depend on the specific sequence of the amino acids. (4)