
Starting around the mid-1700s, electricity seemed to be the gift that kept on giving. Here was a completely mysterious and endlessly fascinating new frontier which had not been penetrated, and continued experiments yielded amazing results. These are the circumstances in which two Italian scientists--Luigi Galvani and Alessandro Volta--had begun their studies of electricity near the close of the 18th century. Actually, before that time, Galvani had been a physician; he attended university for four years to study medicine, graduating in 1759. Around 1780, he was compelled to start investigating the effects of electricity on (dead) creatures and animals. Several mostly apocryphal stories exist about the origins of his foray into bioelectricity. The first one is that, during some experiments with frog's legs, he tied up the legs with copper wire and hung them on a metal window in his laboratory. To his immense surprise, the legs began to flop rapidly, as they do in the presence of electricity. The second story is that some time in 1786, Galvani's wife fell ill and her physician prescribed her a diet of frog leg broth to recover. While preparing the frog leg broth, one of Galvani's assistants accidentally discharged an electrified scalpel and the legs began to flop--the significance of which was recognized not by Galvani, but his wife. In reality, analysis of his lab manuscripts and notes indicates that he knew about the basics of electricity and the electric response of frog legs as early as 1780. However you believe he started, Galvani began his research into the effects of bioelectricity, the field of which he is today recognized as being the forefather.
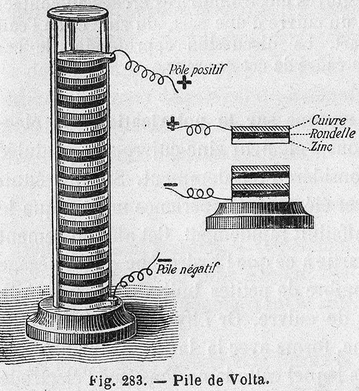
Alessandro Volta, a colleague and occasional rival of Galvani's, would perform, check, and repeat the experiments of Galvani as a sort of second opinion. Galvani correctly recognized the cause of the leg movement as electric charge, but believed that the electricity came from some vital force within the frog. Volta initially took this position until further experiments had him convinced that the source of electricity came from the simple contact between two dissimilar metals (as the copper wire had contacted that iron window frame). Volta set to work comparing contact between metals; he noticed that the contact of some metals produced more charge than others; those metals that were known to easily oxidize were best of all. In addition, he could get better results by moistening the metals with water, or even better, a saline solution. Volta eventually created an interesting construct: a tower of sorts. It was a tower created from a pile of three materials: zinc on the bottom layer, a spongey substance (such as cardboard or cloth) soaked in a saline solution in the middle, and a disk of copper on top. The material would be stacked over and over--zinc, sponge, copper; zinc, sponge, copper; and so forth. To the ends were connected two terminals of wire. Volta confided his invention in a 1796 letter to Sir Joseph Banks in which he described the column and its construction. It was termed the "Voltaic pile" and was in fact the first chemical battery (the term "battery" was first coined by Benjamin Franklin, who did experiments with large numbers of Leyden jars. He collectively referred to the Jars as a "battery", analogous to a battery of artillery cannon.) Whereas the Leyden jar provided large amounts of charge, but was discharged, the Voltaic pile generated small amounts of charge but seemed to recharge itself constantly. The Voltaic pile, like the Leyden jar, was an important discovery because electric scientists now had access to a very convenient, continuous amount of charge with which to experiment.
Most batteries in modern times, like the Voltaic pile, work on chemical action (though at the time, Volta did not recognize it as such, thinking the charge source to be merely the contact between the dissimilar metals of copper and zinc.) Modern batteries, before the advent of rechargable batteries, were composed of zinc and carbon in a mixture of ammonium chloride and zinc chloride. Through a redox chemical reaction, electrons would be extracted from the carbon (which becomes positively charged and thus set as the positive terminal of the battery) and attracted to the zinc (which becomes the negatively charged negative terminal.) The charge seperation would create a potential difference across the terminals of the battery; once a circuit connected the two terminals, charge would flow through the battery terminals due to the electric field caused by their potential difference. The voltage generated by the battery is usually referred to as an "electromotive force" or EMF.
The unit of electromotive force, voltage, potential, potential difference, etc. (they are all the same) is the volt, defined as the amount of potential energy possessed by one coloumb of charge in some electric field, and 1 V = 1 J/C. The volt is, obviously, named for Alessandro Volta.