Photoelectric Effect
The photoelectric effect is a
material's ability to create an electric voltage
or current upon light exposure. PV cells have to
be material with the photoelectric effect. When In
the previous page the amount of electrical power
potential was explored; this page looks at a much
smaller scale where the electromagnetic particles
are interacting with objects of relatively small
surface area. Photovoltaic cells are made of
semiconductor material that has been treated in
order to create an electric field that has a
positive and negative that applies to circuit
terminals. The cell is composed of a selection of
materials in order to create a P-N junction, which
acts as a diode.
Photon Interaction with
Semiconductors
Semiconductor material has a
characteristic called the band gap. The band gap
is a measure of energy in electron-volts (eV) or
Joules. If a photon comes into contact with a
semiconductor the energy level of the photon and
the band gap of the semiconductor will determine
what will happen next. When a photon has a lower
energy than the semiconductor's band gap it passes
through the semiconductor with little effect. For
the semiconductor to produce a charge the photon
that strikes it must have an energy level equal to
or greater than the semiconductor's band gap.
Common semiconductor band gaps are readily
available and the energy level of the photons can
be determined through physics and math.
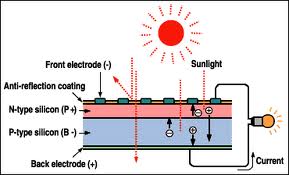
Semiconductors and P-N Junctions
Typical semiconductors are made with
silicon and then the cell is dope to make a P-N
junction that acts as a diode. The diode
characteristic enables the cell to have current
flow in the direction from the positive to
negative, but not the other way around. This
effect of doping to make a P-N junction can be
explained with physics. The basic concept behind
doping is to add elements to the silicon in order
to give it a generally positive or generally
negative potential. Silicon has 4 valence
electrons and when it bonds it creates a
tetrahedral crystal. If material from group III is
added, such as boron, then the 3 valence electrons
from boron bond, but there is a missing bond which
leaves a "hole." The "hole" that is created with
boron doping acts as a positive charge because it
attracts the negative charge. Doping silicon with
boron gives p-type material. When silicon is doped
with an element from group V, such as phosphorus,
then 4 of the valence electrons of phosphorus
bond, but there is one valence electron left that
has the higher ability to flow. Doping silicon
with phosphorus makes n-type material. When the
n-type and p-type material are then placed in
contact some of the available "holes" in the
p-type material move to meet with some of the
available electrons; this bond forms the barrier
which limits flow. Before the barrier cuts off the
flow of current, the n-type material lost
electrons through attraction to the holes and
gives the n-type material a quantitative positive
charge as it lost electrons and this creates an
electric field. Because there is separation
between the two materials there is a potential for
current flow that will not be achieved until a
circuit is completed to both sides.
www.wcubed.com
|

