How
soap and water interact to form a
bubble:
The formation of soap
bubbles is intriguing, because it relies on
the physics of water (which is, in my opinion,
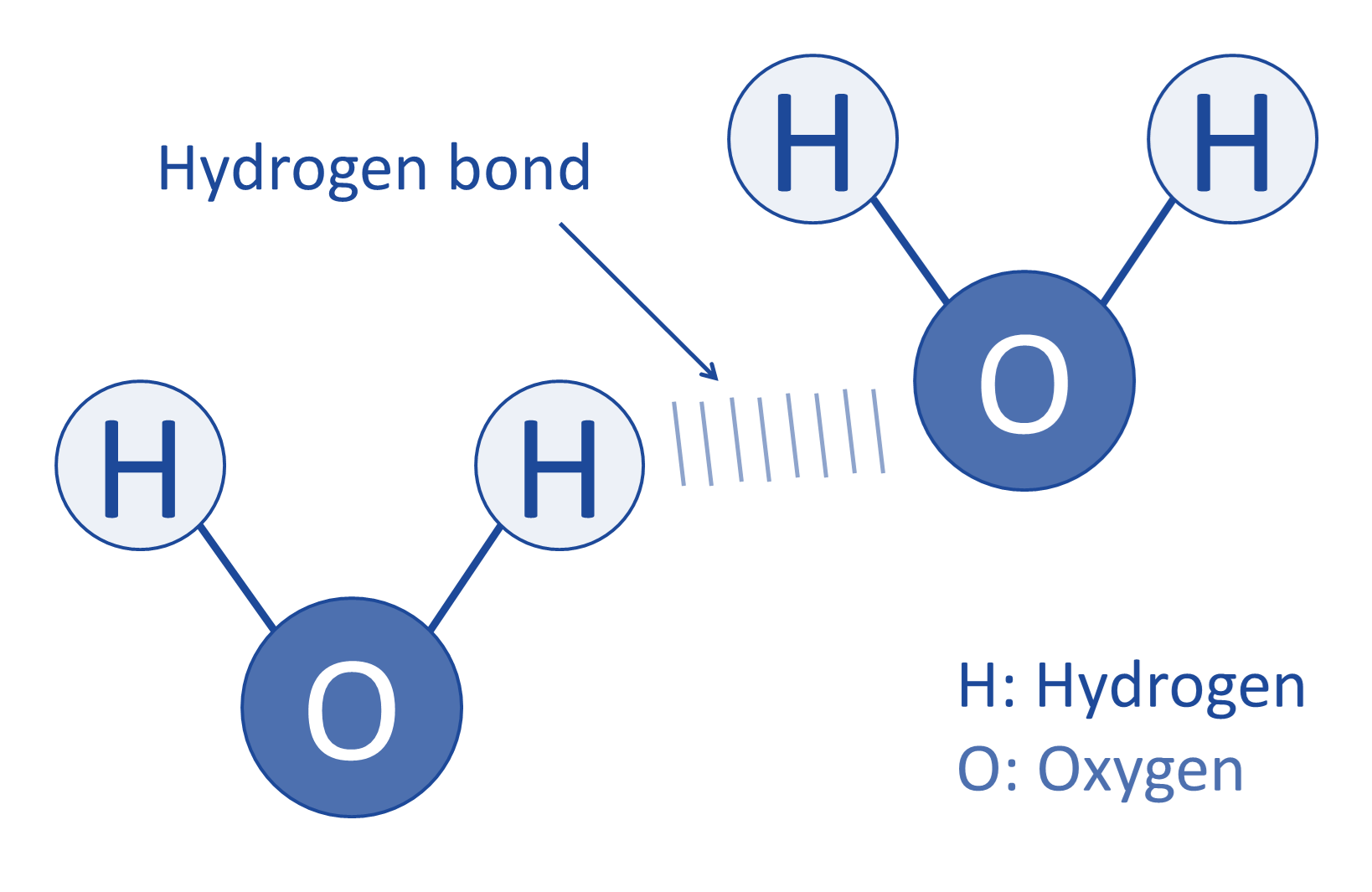
even more fascinating) (4). Water bonds to
itself and each water molecule connects to
another through an attractive force called
hydrogen bonds (Figure 1). Hydrogen bonds
result from an electrostatic attraction
between a proton (from a hydrogen atom) in a
water molecule and an electronegative atom
(oxygen) in another water molecule (5). A
simple experiment to observe hydrogen bonds is
to add as many drops of water possible onto a
dry penny. If gentle enough, one could add
over thirty drops, creating a dome shape of
water on the penny. The dome will not exceed
the width of the penny, but it will keep
getting taller.
For
another experiment, add a little soap to a
bowl of water. It will spread across the
surface forming a layer between the air and
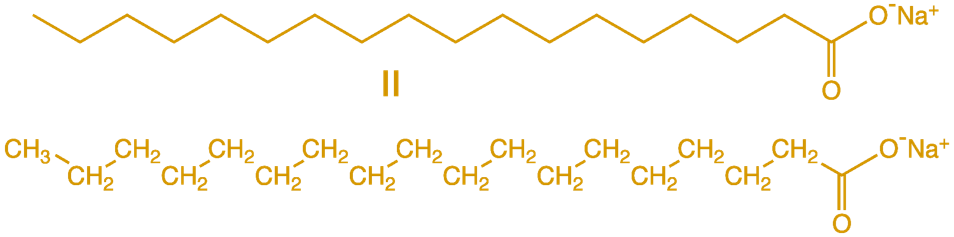
the water (4). This is because of the way soap
molecules are (Figure 2). They resemble a long
worm with many hydrogen and carbon atoms as
its body/tail, and oxygen and sodium atoms as
its head. The tail is hydrophobic, or
“water-fearing,” so it is attracted to oil and
bonds to it. The head is hydrophilic, or
“water-loving,” so it is attracted to water
and pulls the oil free from other oil
molecules (6).
The
hydrophilic head also weaken water’s
attraction to itself. This allows soap to
clean most things and it’s how bubbles form.
With soaps
hydrophilic and hydrophobic ends, a “trio of
layers” is created (Figure 3). It is
essentially a top and bottom layer of soap
molecules with water molecules trapped in
between (6). This phenomenon is why soap
bubbles are round and sphere shaped. Also, the
“trio of layers” helps prolong the time of
evaporation by preventing air from entering
and escaping the bubble. However, since
gravity pulls the soap molecules downwards,
the bottom becomes thicker and the top
thinner. This causes the air inside to rush
out, bursting the bubble. 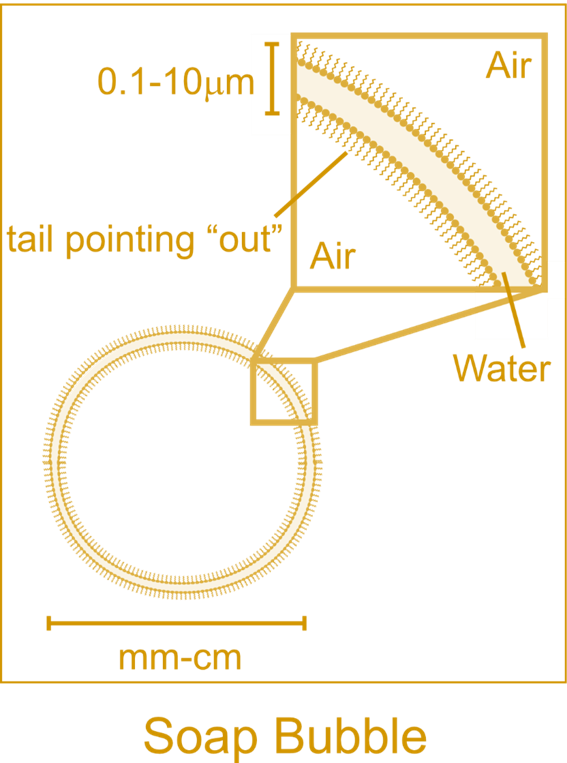 Figure 3. Visual of the "trio of layers." In the zoomed-in area of the bubble, the white part indicates water, and the little yellow hairs pointing away from the water are the hydrophobic tails. (http://www.clearbiology.com/)
Updated 11/23/2016
|