http://dematerialism.net/Appendix%20Ia_files/image014.gif

http://dematerialism.net/Appendix%20Ia_files/image014.gif
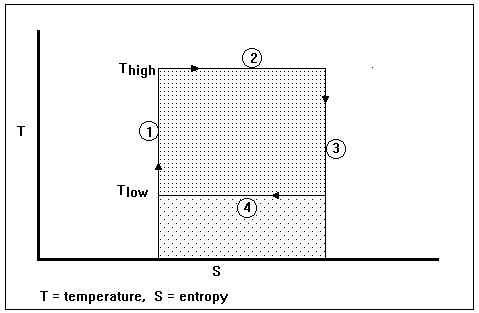
The above diagram represents a Carnot cycle acting as a heat engine. The process is illustrated on a temperature-entropy diagram. The cycle takes place between a hot reservoir at temperature TH and a cold reservoir at temperature TC.
The Carnot cycle when acting as a heat engine (in a piston and cylinder system) consists of the following steps:
Isothermal expansion of the gas at the hot temperature, TH:. Step 2 on the diagram - the expanding gases cause the piston to do work on the surroundings. The gas expansion is propelled by absorption of heat from the high temperature reservoir.
Adiabatic expansion of the gas: Step 3 on the diagram - no heat is gained or lost. The gas continues to expand, doing work on the surroundings. The gas expansion causes it to cool to the cold temperature, TC.
Isothermal compression of the gas at the cold temperature, TC. Step 4 on the diagram. Now the surroundings do work on the gas, causing heat to flow out of the gas to the low temperature reservoir.
Adiabatic compression of the gas: Step 1 on the diagram. The surroundings do work on the gas, compressing it and causing the temperature to rise to TH. At this point the gas is in the same state as at the start of the isothermal expansion.
(Boles)