http://energy.sdsu.edu/testcenter/testhome/Test/intro/introimages/csteady/heatengine.jpg
Carnot Cycle and the Carnot Engine
The Carnot cycle is a special type of thermodynamic cycle. It is special because it is the most efficient cycle possible for converting a given amount of energy into work or for using a given amount of work for refrigeration purposes.
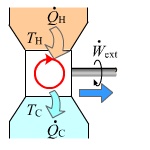
Heat flows from a high temperature TH furnace through the working body (working substance) and into the cold reservoir TC. This process forces the working substance to do work W on the surroundings

http://energy.sdsu.edu/testcenter/testhome/Test/intro/introimages/csteady/heatengine.jpg
No engine operating between two heat reservoirs can be more efficient than a Carnot engine operating between the same reservoirs.
No engine operating between two heat reservoirs can be more efficient than a Carnot engine operating between the same reservoirs. A Carnot Cycle is the most efficient cycle possible in nature. The efficiency η is defined to be:
η = ΔW/ΔQH = 1 – TC/TH
where:
ΔW is the work done by the system (energy exiting the system as work)
ΔQH is the heat put into the system (heat energy entering the system)
TC is the absolute temperature of the cold reservoir
TH is the temperature of the hot reservoir.
The above equation gives the maximum efficiency possible for any engine using the corresponding temperatures. The efficiency can never equal one however. It is not possible as defined by the laws of thermodynamics.