:
http://www.grc.nasa.gov/WWW/K-12/airplane/thermo0.html
There are 3 laws of thermodynamics: the zeroth law, the first and second laws of thermodynamics.
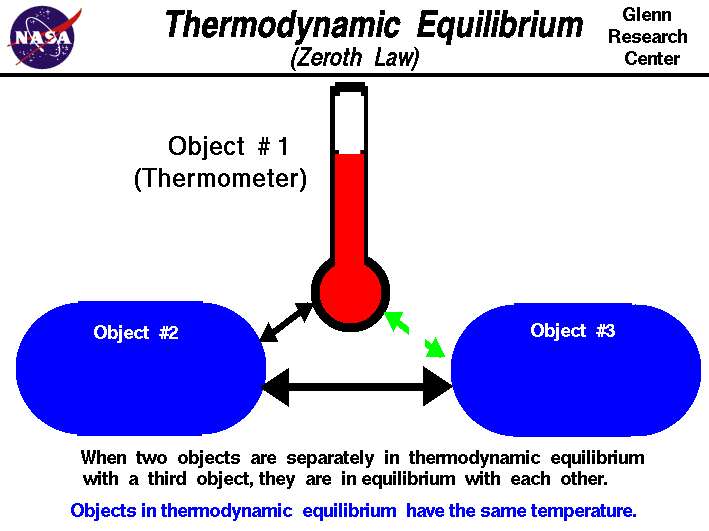
The ZEROTH LAW states the following
:
http://www.grc.nasa.gov/WWW/K-12/airplane/thermo0.html
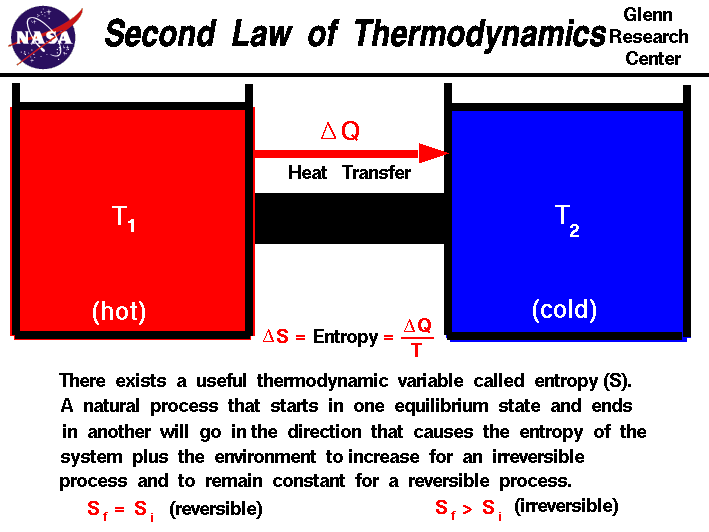
The FIRST LAW of thermodynamics is about conservation of energy. The energy can never be created or destroyed: ΔE = Q + W. The change in the internal energy of a closed thermodynamic system is equal to the sum of the amount of heat energy supplied to the system and the work done on the system.
http://www.grc.nasa.gov/WWW/K-12/airplane/thermo1.html
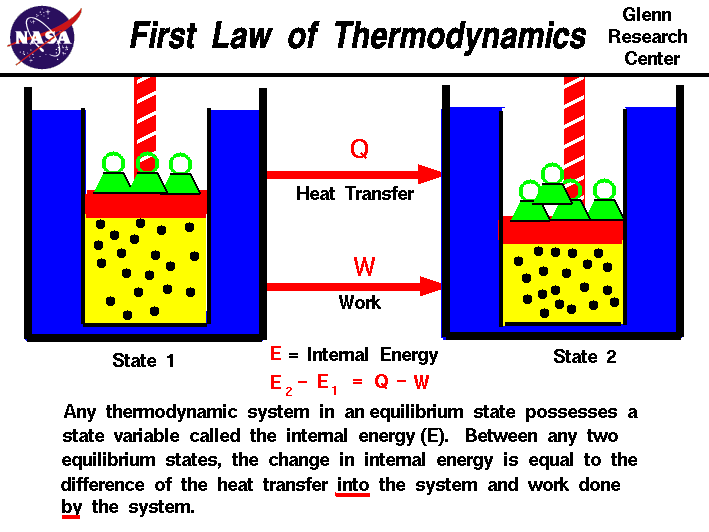
The SECOND LAW of thermodynamics is about entropy. The total entropy of any isolated thermodynamic system tends to increase over time, approaching a maximum value. It also states that the maximum efficiency of an engine is never equal to 1 and the maximum possible efficiency is approached by a Carnot engine.
http://www.grc.nasa.gov/WWW/K-12/airplane/thermo2.html