Physics of Geothermal Energy
The Physics of Geothermal Energy continued
Geothermal Energy production can be explained by the first three laws of thermodynamics, and the efficiency evaluated.
Second Law:
www.grc.nasa.gov
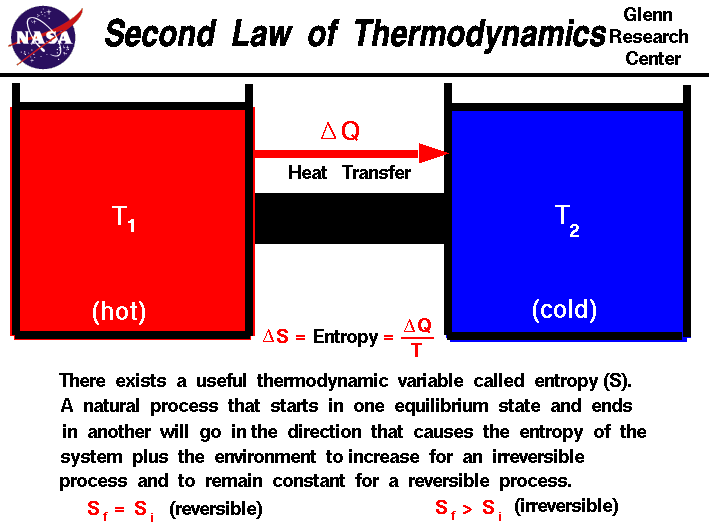
The second law of thermodynamics states:
If a process happens in a closed system, the entropy of that system increases for an irreversible process. If the process is reversible, the entropy is constant. For any process in a closed system, the entropy NEVER decreases.
In the case of geothermal energy, we can assume that the system between the ground, the mixing chamber, and the turbine is a closed system. This can be assumed because the process of pumping up the water and returning it to the earth requires a fair amount of energy that cannot be regained. If this were not the case, geothermal energy production would be a perpetual system and would be much wider spread.
Efficiency of a Geothermal Process:
Now that it is clear that the first three laws of thermodynamics can be applied to the process, it is also relevant to know the efficiency. By the first law of thermodynamics, an energy balance is required. This means that the energy of the compressing stage has to equal the energy produced plus any loss of work due to evaporation. This is modeled as
Qevap + Pdrive = Qcomp.
Where Qevap is the loss due to evaporation, Pdrive is the compressor drive power, and Qcomp is the heat of the compressor. After manipulating the equation, the efficiency can be defined as the ratio of the output to the input, or by the equation
Efficiency = Qcomp./Pdrive = Qevap + Pdrive / Pdrive or
Efficiency = 1 + Qevap/Pdrive (Renewable Energy, p.392)
Geothermal Energy production can be explained by the first three laws of thermodynamics, and the efficiency evaluated.
Second Law:

www.grc.nasa.gov
The second law of thermodynamics states:
If a process happens in a closed system, the entropy of that system increases for an irreversible process. If the process is reversible, the entropy is constant. For any process in a closed system, the entropy NEVER decreases.
In the case of geothermal energy, we can assume that the system between the ground, the mixing chamber, and the turbine is a closed system. This can be assumed because the process of pumping up the water and returning it to the earth requires a fair amount of energy that cannot be regained. If this were not the case, geothermal energy production would be a perpetual system and would be much wider spread.
Efficiency of a Geothermal Process:
Now that it is clear that the first three laws of thermodynamics can be applied to the process, it is also relevant to know the efficiency. By the first law of thermodynamics, an energy balance is required. This means that the energy of the compressing stage has to equal the energy produced plus any loss of work due to evaporation. This is modeled as
Qevap + Pdrive = Qcomp.
Where Qevap is the loss due to evaporation, Pdrive is the compressor drive power, and Qcomp is the heat of the compressor. After manipulating the equation, the efficiency can be defined as the ratio of the output to the input, or by the equation
Efficiency = Qcomp./Pdrive = Qevap + Pdrive / Pdrive or
Efficiency = 1 + Qevap/Pdrive (Renewable Energy, p.392)