Physics of Geothermal Energy
The Physics of Geothermal Energy
Geothermal Energy production can be explained by the first three laws of thermodynamics.
Zeroth Law:
www.grc.nasa.gov
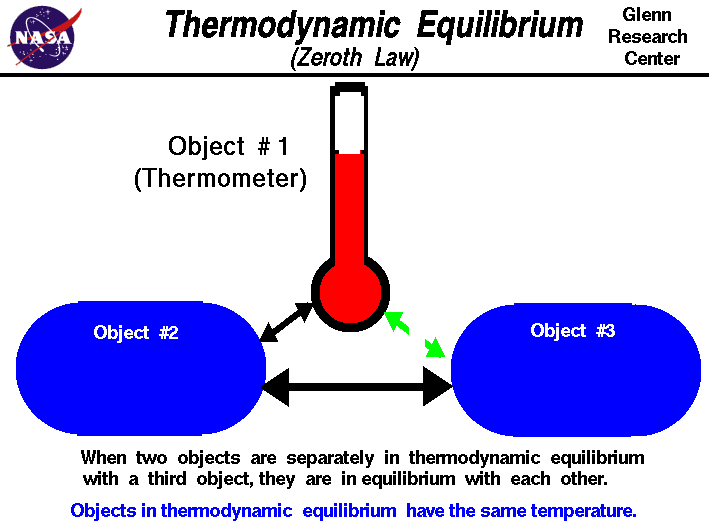
The zeroth law of thermodynamics states:
If two bodies are in thermal equilibrium with a third body, then they are in equilibrium with each other
In the case of geothermal energy, this can be used to explain the process of the vaporization of the refrigerant. The water and the refrigerant are not in thermal equilibrium at first, so anything in contact with them will be at a different temperature. The heat will flow from the hot water to the refrigerant until those two bodies are in thermal equilibrium.
The heat will flow because "Heat is the energy transferred between a system and its environment because of a temperature difference that exists between them." (Halliday and Resnick, p. 484)
First Law:
www.grc.nasa.gov
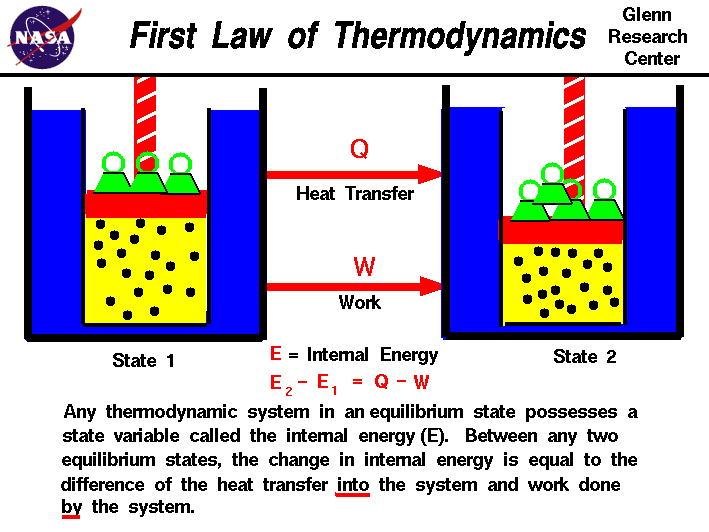
The first law of thermodynamics states:
The internal energy of a system will increase if heat is added and decrease if work is done by the system
This law is also the conservation of the internal energy of a system. In the case of geothermal energy, heat is added to the system of the refrigerant and turbine by the temperature difference between the refrigerant and the water. If heat is added, the internal energy of the system must either increase, or be offset by work being done. Thus the turbine will provide the work done by the system. E = Q - W will be satisfied and energy conserved.
Geothermal Energy production can be explained by the first three laws of thermodynamics.
Zeroth Law:

www.grc.nasa.gov
The zeroth law of thermodynamics states:
If two bodies are in thermal equilibrium with a third body, then they are in equilibrium with each other
In the case of geothermal energy, this can be used to explain the process of the vaporization of the refrigerant. The water and the refrigerant are not in thermal equilibrium at first, so anything in contact with them will be at a different temperature. The heat will flow from the hot water to the refrigerant until those two bodies are in thermal equilibrium.
The heat will flow because "Heat is the energy transferred between a system and its environment because of a temperature difference that exists between them." (Halliday and Resnick, p. 484)
First Law:

www.grc.nasa.gov
The first law of thermodynamics states:
The internal energy of a system will increase if heat is added and decrease if work is done by the system
This law is also the conservation of the internal energy of a system. In the case of geothermal energy, heat is added to the system of the refrigerant and turbine by the temperature difference between the refrigerant and the water. If heat is added, the internal energy of the system must either increase, or be offset by work being done. Thus the turbine will provide the work done by the system. E = Q - W will be satisfied and energy conserved.