 In
1908, Dutch Physicist Heike Kammerlingh Onnes became the first man to cool
helium to its liquid state, 4K. Though he initially only liquefied a few milliliters,
it opened up new doors to low-temperature experimentation. In 1911, he began
to experiment with many metals at low temperatures. It had been known that
the resistivity of a metal decreased as its temperature decreased, but it
was unknown whether it would decrease indefinitely or reach some small limiting
value. Some scientists, William Kelvin included, believed that the flow of
electrons would cease as the conductor approached absolute zero. Onnes took
a rod of very pure mercury and measured its resistivity as he gradually reduced
its temperature. To his surprise, once the temperature reached a certain point,
the resistivity vanished completely! As he
In
1908, Dutch Physicist Heike Kammerlingh Onnes became the first man to cool
helium to its liquid state, 4K. Though he initially only liquefied a few milliliters,
it opened up new doors to low-temperature experimentation. In 1911, he began
to experiment with many metals at low temperatures. It had been known that
the resistivity of a metal decreased as its temperature decreased, but it
was unknown whether it would decrease indefinitely or reach some small limiting
value. Some scientists, William Kelvin included, believed that the flow of
electrons would cease as the conductor approached absolute zero. Onnes took
a rod of very pure mercury and measured its resistivity as he gradually reduced
its temperature. To his surprise, once the temperature reached a certain point,
the resistivity vanished completely! As he 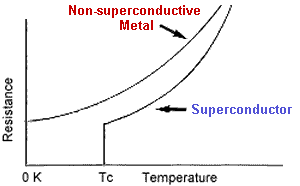 noted,
"Mercury has passed into a new state, which on account of its extraordinary
electrical properties may be called the superconductive state." Onnes
was awarded the Nobel prize for this discovery in 1913.
noted,
"Mercury has passed into a new state, which on account of its extraordinary
electrical properties may be called the superconductive state." Onnes
was awarded the Nobel prize for this discovery in 1913.
Resistivity in Normal Conductors
In order to understand superconductivity, it's helpful to understand some of the basic properties of electricity in normal conductors. Without a difference in potential, there is no net flow of electrical charge in a conductor. Once a potential is introduced across a wire, an electric field is established, and electrons are "pushed" by this field. The electrons flowing through a conductor in such a way is called a current. However, these electrons do not move unimpeded; they collide and interact with the atoms of the conductor. These collisions and interactions impede (or resist) current flow; the collective result of which is called resistance.
