| Sea ice is frozen sea water. Salt ions in the water complicate the growth of ice crystals, and makes sea ice much more dynamic than freshwater ice. Sea ice covers nearly 7% of the Earth's surface, has a huge effect on global climate, and is one of the largest, single biomes on Earth. | |||
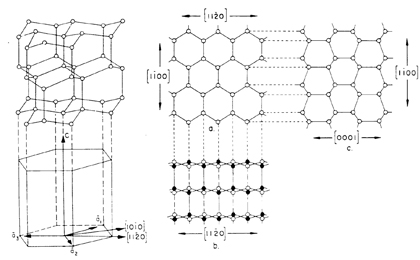
| Ice is the solid, crystalline form of water, which solidifies at 0ºC. Roughly 9 polymorphs of ice are defined, only one, however, occurs naturally on Earth. This common form of ice is known as ice 1h, and its lattice displays six-fold rotational symmetry. | |||
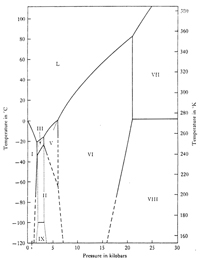
Phase Transition Diagram for Ice (Hobbs, 1974) |
Crystal Structure of ice 1h (Weeks and Ackley, 1986) |
||
| The incorporation of sea salt or other ions in the crystal lattice of ice faces both size and charge restrictions, thus the salt and water do not form solid solution. This means that as the ice grows, the ions are rejected and most of them are returned to the water. Some, however, are retained within the ice matrix as liquid inclusions; creating a network of channels through with this brine travels. | |||
|
|||
| The network of channels and associated brine inclusions greatly contribute to what makes sea ice different from freshwater ice. These inclusions change the deformational, thermal, and optical properties of ice; making sea ice unique in several different ways. | |||
Below are two thin sections of ice, each between two sheets of polarizing film, oriented at 90º. |
|||
Freshwater Ice |
Sea Ice |
||

|
 |
||
Note the difference in grain size, as well as the intensity of the interference colors. |
|||