|
What is Fission? |
|
|||
|
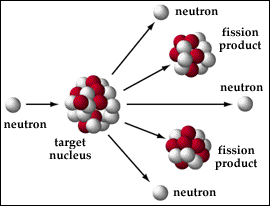
The nucleus of a uranium-235 atom consists of 92 protons and 143 neutrons. When a U-235 atom absorbs an extra neutron it becomes unstable and will fission. The products of the splitting of the nucleus, the largest of which are called fission products, have a total mass that is less than the original mass of the uranium atom. The lost mass is converted into energy. Einstein said in his famous equation E=mc2 that energy and mass are inter-changeable. You can convert mass to energy and energy to mass and as you can see with fission you convert mass into energy. Not all forms of uranium are fissionable, U-238 for example is not fissionable, but it can absorb neutrons and form plutonium-239, which is fissionable. And in a nuclear power plant almost half of the heat energy produced comes from P-239, even if there is none of it to begin with. Nuclear fission can be used for many things including: nuclear weapons, it is used to detonate thermonuclear weapons; nuclear reactors that power ships, as well as to start fusion power plants and fuel nuclear power plants.
|
||||