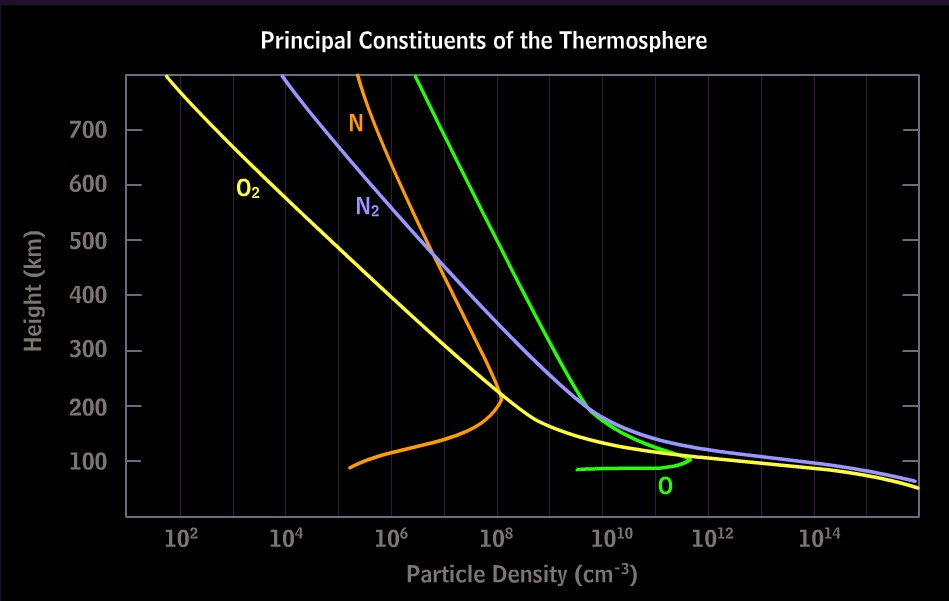
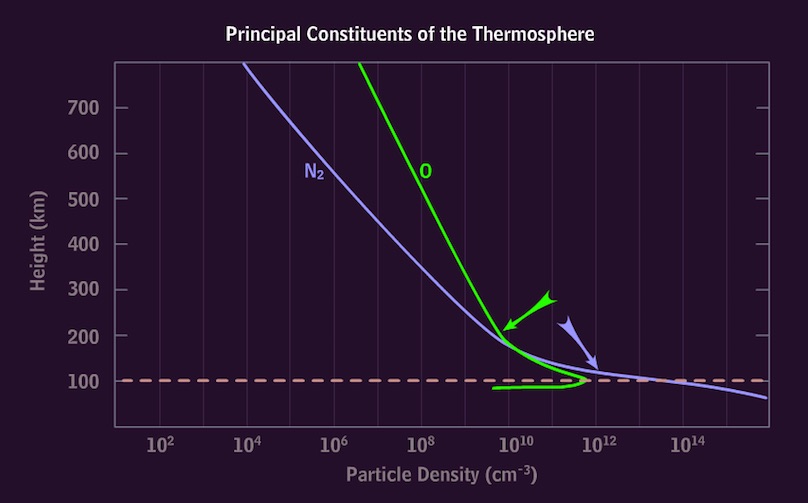
MetEd/Comet
http://www.meted.ucar.edu/hao/aurora/aurora.1.htm
(free membership; Physics of the Aurora - Earth Systems; Aurora Section 2.2
MetEd/Comet
http://www.meted.ucar.edu/hao/aurora/aurora.1.htm
(free membership; Physics of the Aurora - Earth Systems; Aurora Section 2.1
MetEd/Comet
http://www.meted.ucar.edu/hao/aurora/aurora.1.htm
(free membership; Physics of the Aurora - Earth Systems; Aurora Section 2.2