Dr. Somorjai's Contributions to
Solid Surface Physics
~Margaret Yngve ~ Phys 212 Fall 2009~
 In the past, it was argued that as skates passed over ice, they applied pressure and friction to the ice, which heated up a layer of it, and created a water layer that acted as a lubricant, and allowed the skates to ride on it on a frictionless surface. This theory, although still widely believed, has been recently disproven by Dr. Gabor Somorjai, a chemist at UC Berkeley. In the last 10-15 years, Dr. Somorjai and his crew have run multiple tests on ice, and discovered that while you do melt some of the ice as your skates pass over it, it is not necessary for the skates to glide. Because of the polar nature of water, it exists in molecular form with one awkward oxygen hanging out on the edge of the surface. If this structure was in a crystal lattice (frozen solid structure, the red oxygen atoms would be on the outside of the surface due to their polar nature.
In the past, it was argued that as skates passed over ice, they applied pressure and friction to the ice, which heated up a layer of it, and created a water layer that acted as a lubricant, and allowed the skates to ride on it on a frictionless surface. This theory, although still widely believed, has been recently disproven by Dr. Gabor Somorjai, a chemist at UC Berkeley. In the last 10-15 years, Dr. Somorjai and his crew have run multiple tests on ice, and discovered that while you do melt some of the ice as your skates pass over it, it is not necessary for the skates to glide. Because of the polar nature of water, it exists in molecular form with one awkward oxygen hanging out on the edge of the surface. If this structure was in a crystal lattice (frozen solid structure, the red oxygen atoms would be on the outside of the surface due to their polar nature.
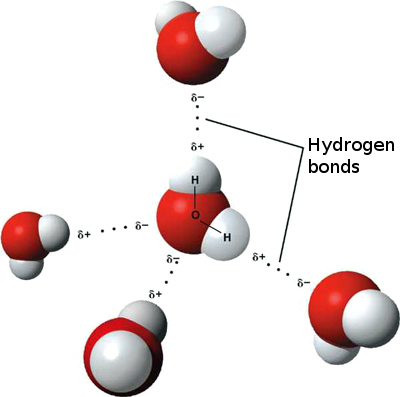 When these oxygens vibrate, as they do because of their atomic energies, they create a frictionless gliding surface. This perfect, one molecule thick gliding surface exists at -250 Fahrenheit, but it gets thicker as ice warms up. This explains the higher friction in warmer, slower ice! There are more Oxygens slowing you down!
When these oxygens vibrate, as they do because of their atomic energies, they create a frictionless gliding surface. This perfect, one molecule thick gliding surface exists at -250 Fahrenheit, but it gets thicker as ice warms up. This explains the higher friction in warmer, slower ice! There are more Oxygens slowing you down!