Image Courtesy Of: Compadre
Plasma.
Charged
Gases.
Elements.
Solar
Winds.
Atmosphere.
Density.
Photons. Excitement.
Spectrum.
Wavelength.
COLORFUL
AURORA!
The secret to earth's
vivid aurora has been fundementally entrusted in two elements:
nitrogen
and oxygen. These two gases, and they alone, hold the colorful
secret of our aurora's flashy hues.
Image
Courtesy
Of:
Ask
Harry
Gilbert
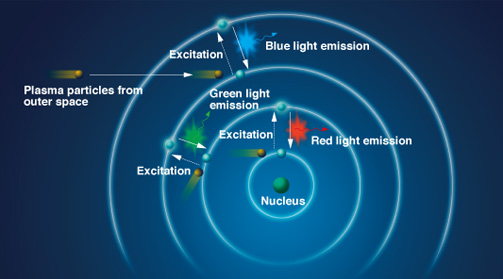
The production of light
is an elegant matter of chemistry and physics. In essence, light is
created by the release of energy from an excited atom. To excite an
atom, charged particles increase the energy of an atom, while
simultaneously decreasing its stability. To return to a more stabilized
state, atoms get rid of their excess energy by releasing it in the form
of light. The energy given off by the light emission is known as a
photon, a term scientists use to describe a single "packet" of light.
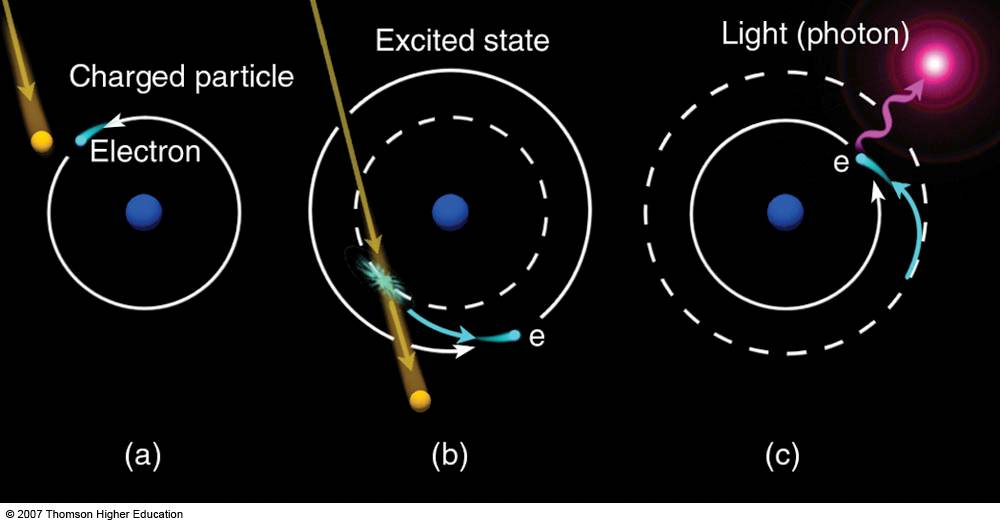
Image Courtesy
Of: Vermont
State
College
The
color
of
emitted
light
depends on the energy of the atom at the time the energy was
released. As electrons orbit an atom's nucleus, an atomic collision
with a charged particle is able to make the electrons jump from one
orbit into another. The greater the jump—as
dictated
by
the
collision
strength between the atom and charged particle—the
more
excited
the
atom
becomes.
The chart below
displays an example of the relationship between atoms in the atmosphere
and the collision strengths required to produce specific color
emissions.
Image Courtesy
Of: Nikon
Nitrogen and oxygen
atoms, due to their different densities, tend to form varying elemental
concentration
groups at different heights in the atmosphere. Because each of the
gases give off a unique spectrum—a range of color
corresponding to a range
of
collision strengths—the different
concentrations of these
spectrums at
every level of the atmosphere provide us spectacular blends of color as
far as the eye can see.
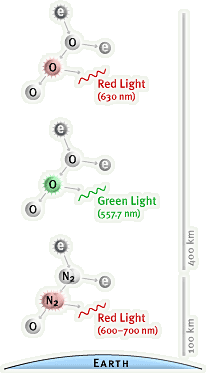
Image Courtesy
Of: Web
Exhibits
The
above
image
provides
a
visual example of coloration from oxygen and nitrogen reactions at
different heights above the earth's surface.
Below is
a spectrum for
blends of nitrogen and oxygen.

Image Courtesy
Of: Web
Exhibits
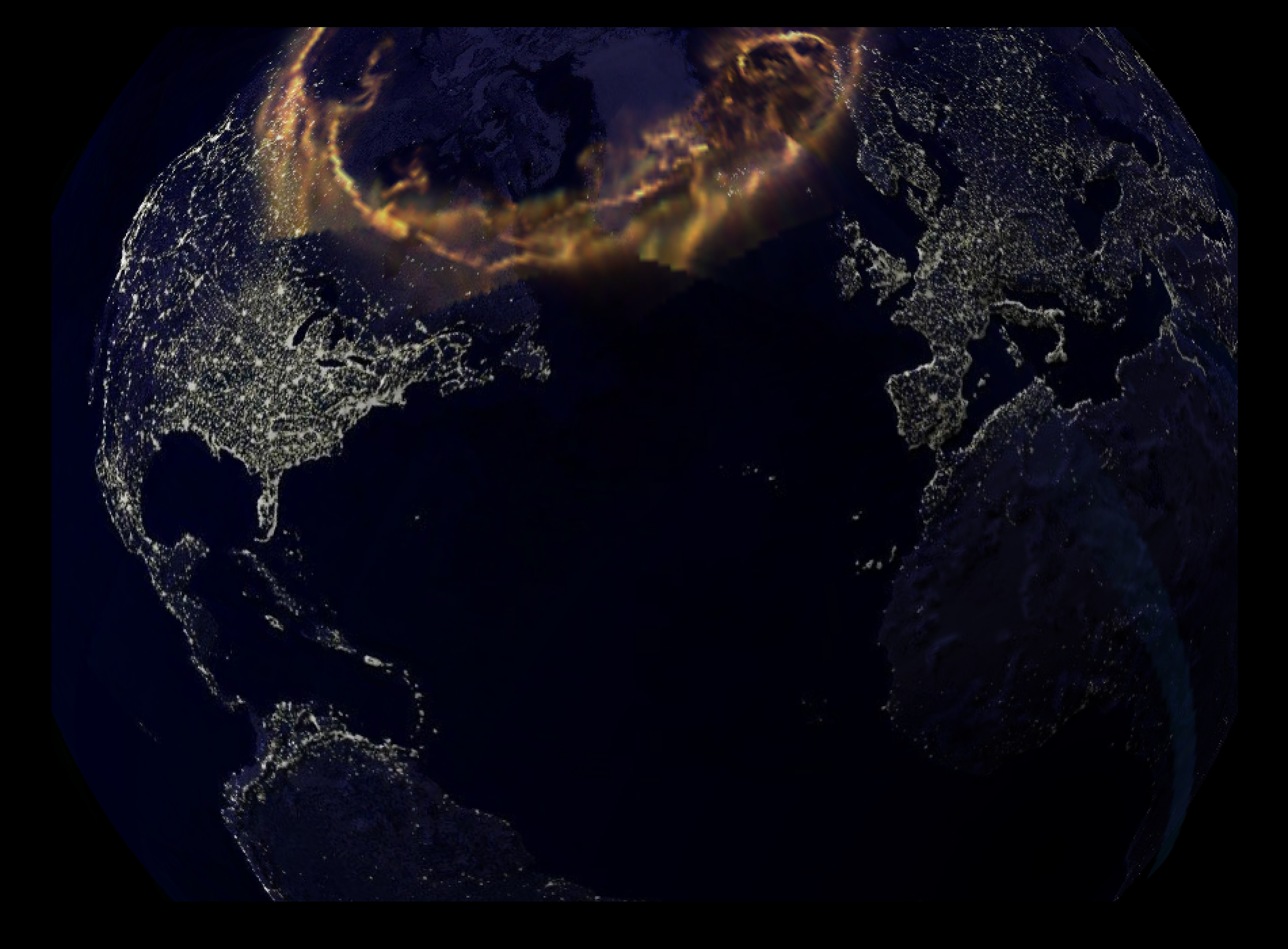 Image Courtesy Of: John
Hopkin's
University
Image Courtesy Of: John
Hopkin's
University