How would we distinguish them
In this golden age of atomic physics an
obvious question was given serious scientific thought, atoms
are small fast and hard to see. There is a fundamental rule in
physics that you cannot absolutely pin down the location of an
electron at any given time, this comes from the Heisenberg
uncertainty principle stated below.
This formula states that there must be
uncertainty in either the wavelength or the location of an
electron. Due to the fact that we cannot pin down the location
of any one wavelength or location together we have no way to
'prove' that any electron is separate than the one next to it
at a given second. The formula above has been verified again
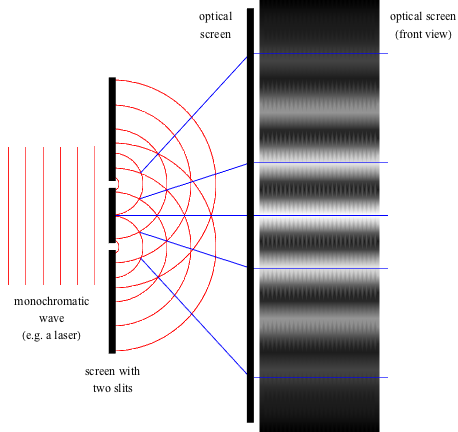
and again in double slit experiments. As the commonly seen
interference pattern in the experiment is a direct result of
the the uncertainty in momentum. Image courtesy of https://www.shmoop.com/optics/young-double-slit.html.
Another thing to note is that every electron is identical
in both charge and mass. This part is actually been known
for a long time. The fact that the charge is uniform is how
you are reading this computer. Electrons being uniform in
mass is actually a key factor in how basic wiring operates.
Electricity basically works by pushing electrons through a
wire, electron charge over time is how we define current. If
electrons weren't uniform in charge current would be
unpredictable and difficult, possible dangerous to harness.
The mass of an electron can be determined by shooting it at
atoms, measuring where it goes and applying kinematic
equations among other ways. These ideas have been known for
a while and have been tested thousands of times. As far as
we can tell every electron is exactly uniform.