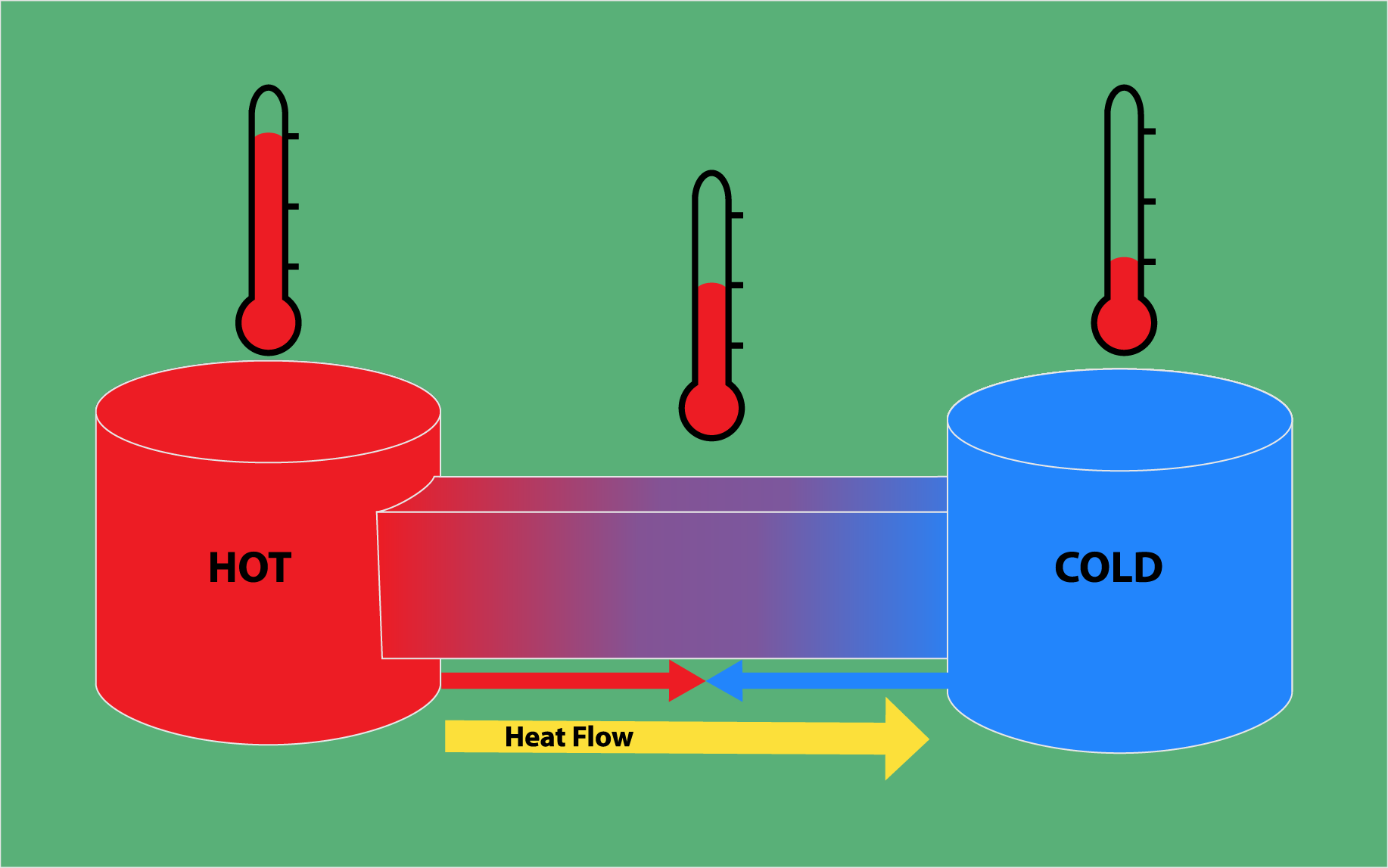
Heat
Heat is
often associated with things being hot, but heat in
physics is just the transfer of energy between two
objects because of their temperature difference.
The way that energy is transferred is from hot to
cold. The molecules in a hot object are moving a
lot faster than the molecules in the cold object,
and so the fast moving molecules interact and
collide with the slower molecules, transferring
energy to them. This means that the hotter object
(with faster moving molecules) is losing energy to
the cooler object.
|
|

