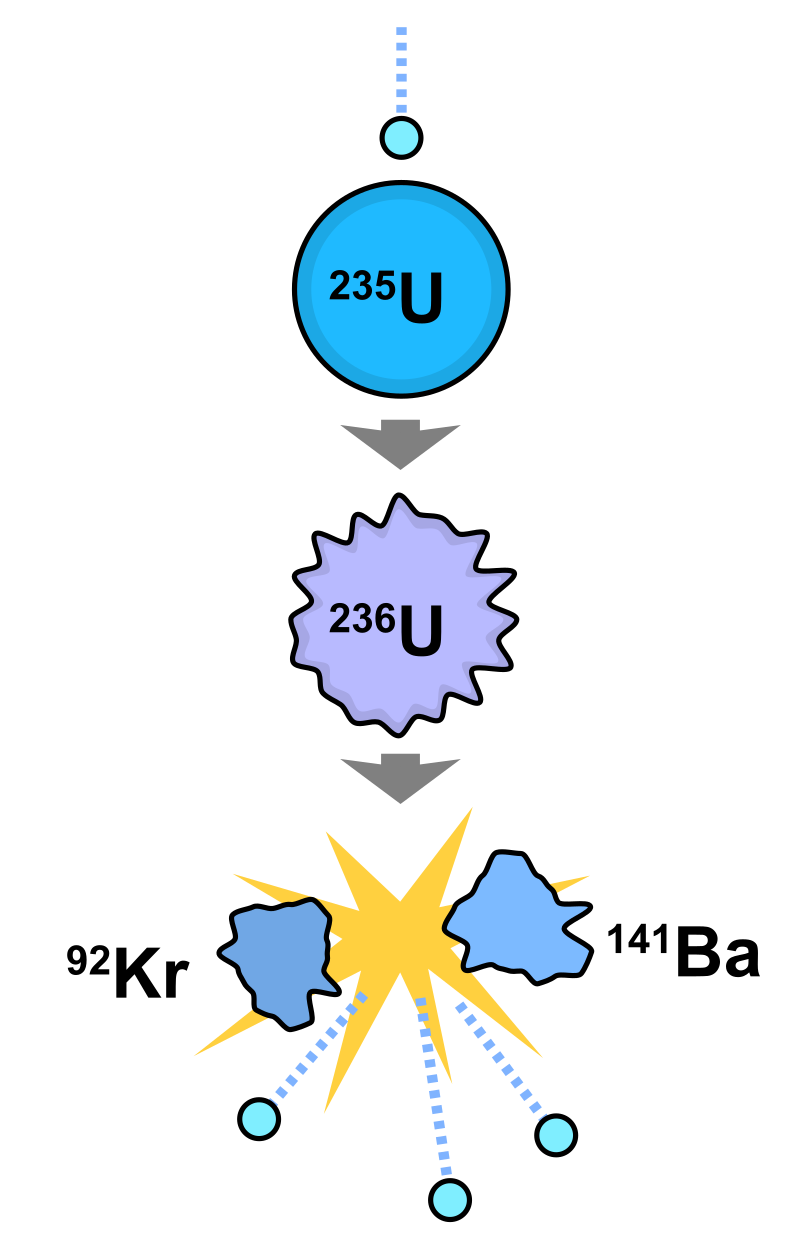
At the core of what drives the energy creation of nuclear reactors is a process known as nuclear fission. Nuclear fission inside a nuclear reactor occurs when a stray neutron is fired at an atom and then "captured" into the nucleus. The additional neutron can cause the atom to become unstable and split into lighter elements, releasing energy in the process. This is called nuclear fission. But how does it occur?
As a neutron passes the nucleus of the atom, the neutron and the protons within the atom are pulled together. This pulling force is caused by the strong nuclear force which is a force that attracts nucleons (protons and neutrons) together. The force occurs due to the transfer of subatomic particles known as mesons between nearby nucleons. If the neutron passes the nucleus of the atom close enough this force can cause the capture of the neutron.
In most nuclear reactors, nuclear fission is conducted on a fuel source of uranium-235. The nucleus of uranium-235 has 92 protons and 143 neutrons. This configuration happens to be unstable, which means that uranium-235 readily splits upon the capture of a neutron. This split releases energy that is stored in the bonds between the nucleons in the atom's nucleus. The amount of this energy, called binding energy, released upon each fission event varies, but on average
3.2 x 10-11 joules of energy is produced1.
3.2 x 10-11 joules may seem like an insignificant amount of energy, but along with energy each fission event releases 2-3 additional neutrons which can go on to cause other fission events, eventually leading to a chain reaction. A chain reaction can begin to occur when a reaction becomes critical, meaning when the number of neutrons produced equals the number lost.