Freezing, Melting and
Boiling Point
http://cnx.org/contents/oSrOCkyf@4/Phase-Changes
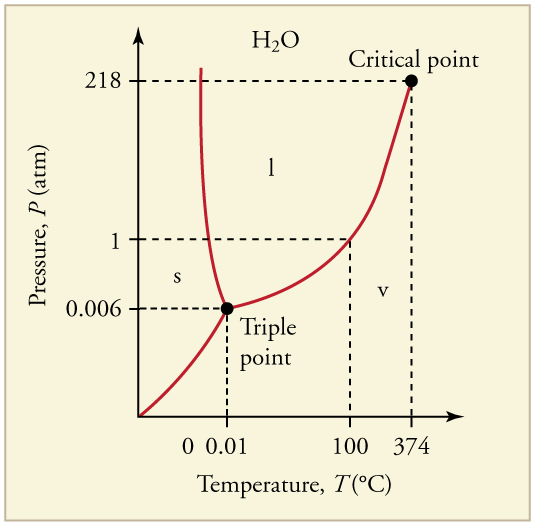
Water is special
because it can have three states of matter exist
in equilibrium at one single temperature. This
circumstance is called a triple point, where the
phase change lines meet up at one point on a
Pressure vs Volume graph. The temperature where
the triple point of water is said to be 0.00
degrees Celsius. The triple point of water is used
to define the kelvin, the base unit of
thermodynamic temperature in the International
System of Units (SI). "The triple point of water
is the standard fixed-point temperature for the
calibration of thermometers. This agreement also
sets the size of the kelvin as 1/273.16 of the
difference between the triple point temperature of
water and absolute zero" (). All three states of
matter can exist in equilibrium because of vapor
pressure.
Vapor pressure is defined
as the pressure at which a gas coexists with its
solid or liquid phase. It can also be defined as
the pressure a gas would create if it occupied the
total volume available. Dalton's law of partial
pressures says that in a mixture of gases, each
gas will exert its own pressure on the other gases
in a confined volume. The total sum of the
pressure is the sum of all individual partial
pressures.

http://www.nuclear-power.net/nuclear-engineering/materials-nuclear-engineering/properties-of-water/triple-point-of-water/
|