 http://www.frostytech.com/articleimages/periodic_graph.gif
http://www.frostytech.com/articleimages/periodic_graph.gif
Mark Underwood Physics 212 March 2017
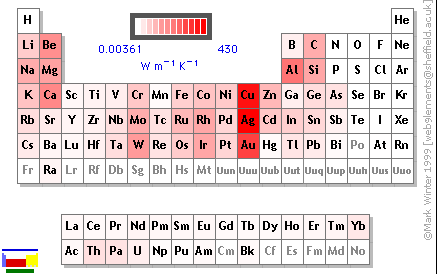
The reason that thermoelectric effects can occur is a property called conductance, which is a material's ability to transmit heat or electricity through itself. This is opposed to insulation, which is a material's inability to transmit heat or electricity. Conductance is a property common to most metals on the periodic table as well as a few other elements. The elements shaded the most red in the picture on the right are the most conductive, white elements being insulators.
The reason that metals are the most conductive elements is an atomic structure called the electron sea model. In this model, the outer electrons of a metal's atoms are allowed to move about freely. The electrons move in a way similar to a gas or a liquid, forming a sort of "sea" in between atoms. These atoms are called ion cores, because removing their outer electrons caused them to become positively charged ions.
If an electric potential is introduced, that is, there is some place that the electrons want to go to, they are completely free to move through or even out of the metal. In an electrical circuit, the electrons are pulled from one end of the circuit to the other through a conductive material, typically with some kind of work being done by them on the way.