Light is both a wave and a particle. The wave contains both an electric and magnetic component, perpendicular to each other, and the particle is called a photon. Light can be described by the equation "c = λv". Where c is the speed of light, λ is the wave length, and v is the frequency.
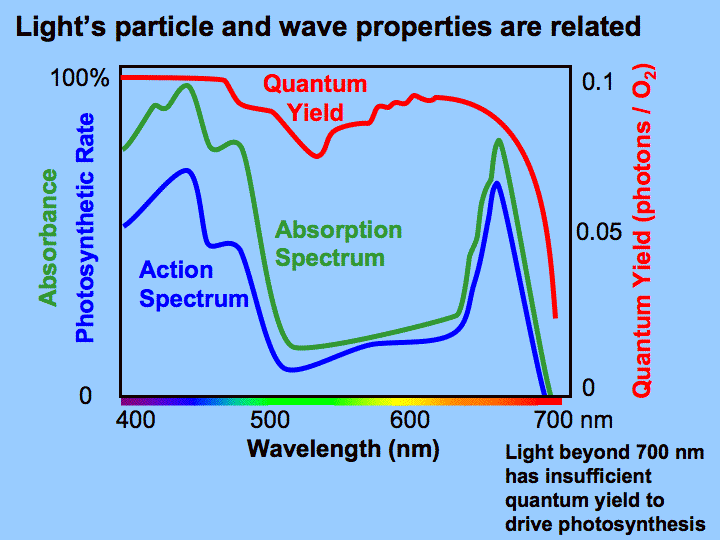
When a photon strikes an electron in the chloroplast the electron is excited. An excited electron is simply an electron that has risen a certain amount of energy levels. This energy being carried by the electron is described by the term quanta, which was first documented by Niels Bohr. If light is being sent to a surface there are two ways to send the energy. First, you can increase the number of photons, each with the same quantum energy level. This increases the intensity, but keeps the frequency, and therefore the energy per photon, fixed. Second, you can increase the energy per photon. This requires increasing the frequency. Plank's constant, h, relates the frequency of a light wave, v, to the amount of energy it carries, E, in the equation: E = hv. This means that there is an optimal wavelength of light that carries the most energy per photon. This is described by the term quantum yield and occurs at the blue (400-500 nm) and red (650-700 nm) portions of the light spectrum. The center of the light spectrum, green light (500-600 nm) is reflected and is the reason most photosynthesizers are green.
Another way plants maximize the amount of light is by implementing the principles of irradiance. An adequate equation is: irridiance = (S) x cos(α). Where S is equal to the total surface area and α is equal to the angle between the light source and a normal vector of the surface. To maximize the amount of light a leaf recieves it must maximize the surface area and ensure that the angle between the light source and the normal vector of the surface is zero, so that the cosine value is maximized as well.