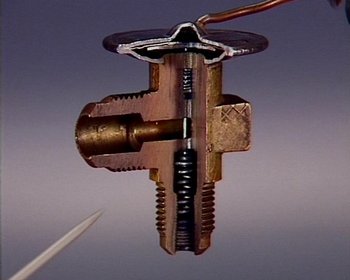
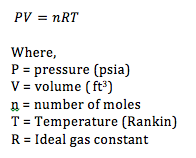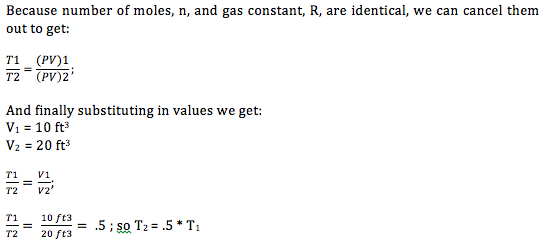The expansion valve is where the cooling takes place. As the refrigerant travels through the valve, it expands. The ideal gas law demonstrates why the fluid cools when it expands. The ideal gas law, which states:
or, temperature two is half that of temperature one.
This means that as the coolant passes through the expansion valve, it experiences a significant decrease in temperature. After the coolant flows through the expansion valve it passes through the freezer (when it’s coldest), and then through the refrigerator. When the liquid coolant hits the low pressure area, it boils and changes into gas (which is called vaporizing). Once the coolant passes through the compartments it heads to the compressor.
This means that as the coolant passes through the expansion valve, it experiences a significant decrease in temperature. After the coolant flows through the expansion valve it passes through the freezer (when it’s coldest), and then through the refrigerator. When the liquid coolant hits the low pressure area, it boils and changes into gas (which is called vaporizing). Once the coolant passes through the compartments it heads to the compressor.