While it may seem
counter-intuitive, the high surface tension of water must be
lowered to allow a bubble to form. This is why bubbles of
only water are very small and short-lived, as they collapse
on themselves. Water also evaporates quickly, which makes
the wall of the bubble become thin very quickly and pop.
By adding soap to water, the surface tension decreases, as soap has roughly one third the surface tension of water. This also helps the bubble live longer, as soap does not evaporate as quickly.
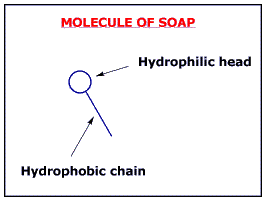
Soap molecules are composed of long chains of hydrogen and carbon, which are arranged in such a way that the head of the chain is hydrophillic, favoring water, and the tail of the molecule is hydrophobic, avoiding water.
By adding soap to water, the surface tension decreases, as soap has roughly one third the surface tension of water. This also helps the bubble live longer, as soap does not evaporate as quickly.
Soap molecules are composed of long chains of hydrogen and carbon, which are arranged in such a way that the head of the chain is hydrophillic, favoring water, and the tail of the molecule is hydrophobic, avoiding water.
http://www.newciv.org/nl/newslog.php/_v434/__show_article/_a000434-000003.htm
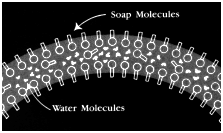
When bubbles
form, the walls essentially have three layers: an outer
layer of soap molecules, a mid-layer of water molecules,
and another layer of soap molecules on the inside.
http://www.exploratorium.edu/ronh/bubbles/soap.html
This structure provides the bubble with enough strength, due to the water's surface tension, and enough resistance due to the soap creating a barrier between the water and air. The surface tension is equal around the surface of the bubble, and a spherical shape requires the least energy to maintain because they have the smallest surface area-to-volume ratio of any structure, so all bubbles move toward spherical shapes.