
What are they?
Semiconductors are material that have the ability to conduct and not conduct current, which is a useful property for producing electronic circuits. This property is useful because computers operate in binary at the lowest level; in 0s and 1s. Those 0s and 1s are simulated physically by semiconductors alternating between conducting and not conducting current. Semconductors are typically made from silicon in the form of crystals.

To understand how semiconductors work, it's important to start with the electron layers of atoms. The most important electron layer when it comes to semiconductors is the outermost layer, called the valence shell. This is where the valence electrons are located, which are the electrons that are readily available to interact with electrons from other atoms.
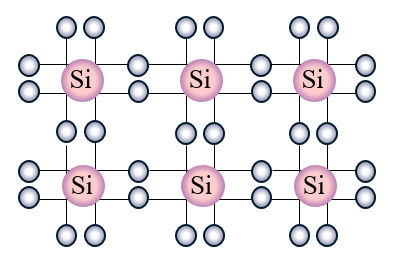
In silicon semiconductors, there are typically four electrons in the valence shell. This number is important when there are multiple silicon atoms grouped closely together because those valence electrons align the atoms together into a crystalline structure. This is how silicon crystals are created. Since each silicon atom typically has four valence electrons, this means each atom is bonded to four other atoms.
It's important to note that silicon crystals by themselves are not very useful in applications like producing semiconductors. In order to change silicon's electrical characteristics to become useful in semiconductors, silicon crystals undergo a process called doping. This process is where a foreign elements are introduced into a crystal in order to change their electrical characteristics.
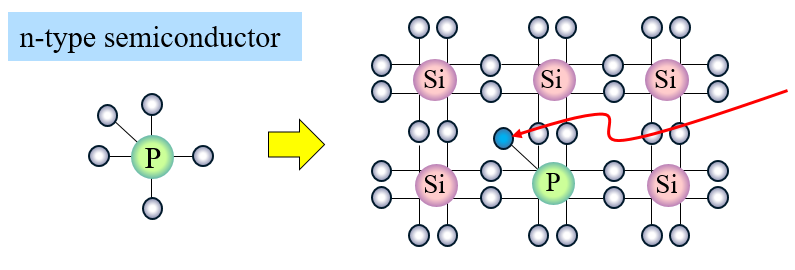
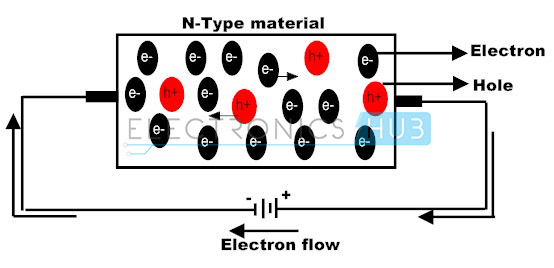
One example of this when an element with five valence electrons is doped into a silicon crystal, which usually ends up being phosphorus. Through this process, an N-type semiconductor is created. While previously, in a pure silicon crystal, all the valence electrons were paired with each other, there will in fact be one unpaired valence electron per phosphorus atom. Consequently, this means there are extra valence electrons able to freely move about, just as the extra valence electrons in metals like copper. It is well known that metals like copper are excellent conductors of electricity, so through this process of doping, the silicon crystal has similarly become an excellent conductor, which is indeed very useful for applications like semiconductors.
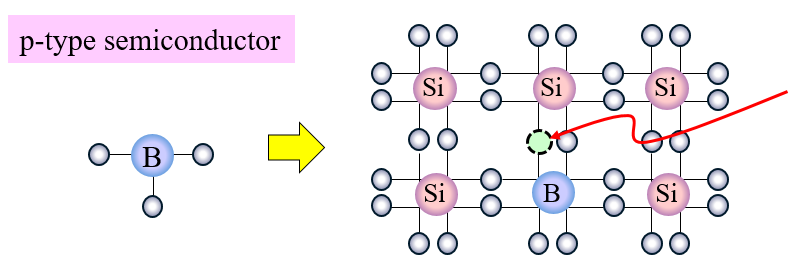
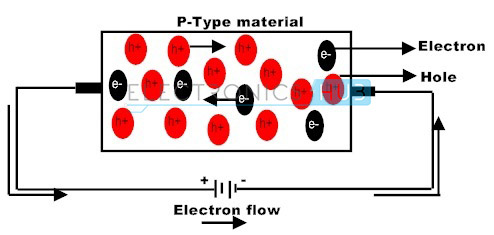
Another example of doping is when an element with three valence electrons is introduced into a silicon crystal. Compared to the perfect pairing of pure silicon in which there are no unpaired electrons, there will be one missing valence electron for every phosporus atom. This is called a hole. These holes act like positive charges in that they attract electrons because they are ready to be filled in to complete bonding. An interesting phenomenon that occurs with these holes is that whenever an electron moves to fill in a hole, the electron leaves a hole where it used to be, thus creating this constant movement of valence electrons within the doped crystal. This is how a P-type semiconductor is created.
Thus, it should be evident that although both N-type and P-type semiconductors are different in what element they introduce to a pure silicon crystal, they both cause the constant movement of valence electrons within the final product. Moreover, when voltage is applied to these semiconductors, it causes current to flow in one organized direction. This is how electric current is created in semiconductors.

How are they created?
The creation of semiconductors is a very involved and complex process, but it can be simplified down to the most important steps.
- Although silicon isn't the best semiconducting material on Earth, it is certainly the most readily available and easy to work with. The most abundant source of silicon is silicon dioxide, also known as sand.
- Sand is collected and converted to a purified, crystalline form through various chemical and physical processes.
- The final product is a large crystalline rod of the highest purity silicon, in which there is only one impurity per every 10 million atoms. These rods of silicon are simply called ingots, are with other purified forms of metals.
- These ingots are then sliced into thin wafers, engraved, processed, and treated in innumerable ways before being cut into square dies for use in processors.
- The laser engraving process is where the perfectly smooth surface of silicon wafers is formed into all the channels and structures that a processor would use to direct and control electrical current.
- All of the dies produced at the end are then packaged in various ways, typically onto PCB substrates that are then integrated into larger circuit boards.




Applications
Since semiconductors are the foundation of computer processors, semiconductors are used in hundreds of unique applications, including:
- Smartphones
- Laptops
- Desktops
- Servers
- Supercomputers
- Cars
- Solar technology
- Medical equipment
- Transistors
- ATMs
- Diodes
- Magnetic field sensors
- MOSFETs
- CMOS
- etc...
As shown, semiconductors are used in numerous applications in our daily lives, whether we know it or not. Since it's been explained what semicodutors are, it's also important to recognize why they are useful in all these applications.
- Due to the vast adundance of sand, silicon semiconductors are economical and mass-produceable.
- They don't require filament heating for operation, unlike vacuum tubes.
- Semiconductor devices are solid-state, meaning they are relatively durable.
- Semiconductor devices are much smaller, cheaper, and more energy efficient than the vacuum tubes they replaced.
- They don't produce any noise during operation.
- They have extremely long lifespans. Under light loads, they can be expected to last over 10 years.
Conclusion
With each passing year, more and more semiconductor devices are being brought into the market to continually improve the quality of life of their users. Although the rate of progress has slowed dramatically in silicon semiconductors over earlier years, semiconductors are here to stay for many more decades and possibly centennials. The concept of semiconductors doesn't restrict them to silicon by any means, and research has been made into alternative materials to replace silicon's use as the base material in semiconductors, such as graphene. The future of semiconductors is bright.
Sources
Information
https://www.extremetech.com/extreme/208501-what-is-silicon-and-why-are-computer-chips-made-from-it
Images
https://image.made-in-china.com/202f0j00vFfQukjsLBzm/Silicon-Wafer-Price-6-Inch-Polished-Wafer.jpg