|
|
Dr. Chadwick's greatest contribution to science. “The discovery of the neutron became an important stage in the understanding of two of the fundamental forces of particle physics, the strong and weak interactions. Neutrons are also known to be important in the life-cycles of stars, especially in the neutron stars or pulsars discovered in 1967.” Dr. Rowland
In 1920 Ernest
Rutherford believed there was another particle
in the nucleus of an atom possibly a proton
electron pair with a net charge of zero, but
never proved that such a particle existed.
This particle was later identified by Chadwick
as the neutron in 1932 and led to him
receiving his Nobel Prize; this discovery was
Chadwick’s greatest contribution to science.
Chadwick replicated experiments of Frederic
and Irene Joliot-Curie but was interested in
finding the 3rd element in an atom.
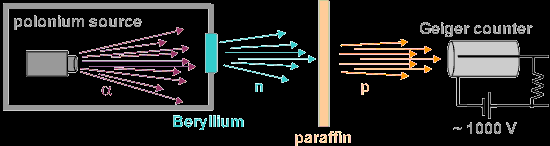
In the experiment he ran alpha particles into
beryllium allowing the beryllium
radiation to collide with paraffin wax, and
then hydrogen atoms hit by the radiation
were sent into a detection chamber.
http://dev.physicslab.org/Document.aspx?doctype=3&filename=AtomicNuclear_ChadwickNeutron.xml
"Using kinematics, Chadwick was able to determine the velocity of the protons. Then through conservation of momentum techniques, he was able to determine that the mass of the neutral radiation was almost exactly the same as that of a proton." Catharine Colwell
Finding the neutron advanced many fields of science from physics to chemistry. The neutron made an ideal bullet in physics because of its neutral charge it could penetrate deep into materials. It was used to split the uranium atom and that allowed for the release of nuclear energy agreeing with Einstein’s famous equation E=mc2. Because of this nuclear fission was possible an application Chadwick would see first-hand when he worked on the Manhattan Project building the atomic bomb.
|
|||||||||||||
| Michael Pritchard PHYS 211X |