Variances in Pressure and Temperature

Pressure and
temperature play an important role in the
formation of minerals. Different minerals form
at a variety of pressures and temperatures.
There are certain minerals that prefer a low
temperature and pressure environment while
others prefer a very high temperature and
pressure environment. In the example below,
the following minerals are polymorphs of each
other. This means that they are the same exact
chemical formula, but they form at different
temperatures and pressures.
"The phase diagram for this system shows
that each mineral is stable over a particular
pressure and temperature range. For
example, andalusite forms only at pressures
less than those of the “triple point”, that
is, the single point in P-T space where all
three phases can coexist. Similarly,
sillimanite can form only at temperatures
greater than that of the triple point.
Additionally, this diagram shows that
progressive heating at low pressure (P <
4000 bars) produces first kyanite, then
andalusite then sillimanite. The
transition from kyanite to andalusite is known
as the andalusite isograd, while the
transition from andalusite to sillimanite is
called the sillimanite isograd. The phase
diagram for this system shows that each
mineral is stable over a particular pressure
and temperature range. For example,
andalusite forms only at pressures less than
those of the “triple point”, that is, the
single point in P-T space where all three
phases can coexist. Similarly,
sillimanite can form only at temperatures
greater than that of the triple point.
Additionally, this diagram shows that
progressive heating at low pressure (P <
4000 bars) produces first kyanite, then
andalusite then sillimanite. The
transition from kyanite to andalusite is known
as the andalusite isograd, while the
transition from andalusite to sillimanite is
called the sillimanite isograd."(Cashman,
Oregon University)
|
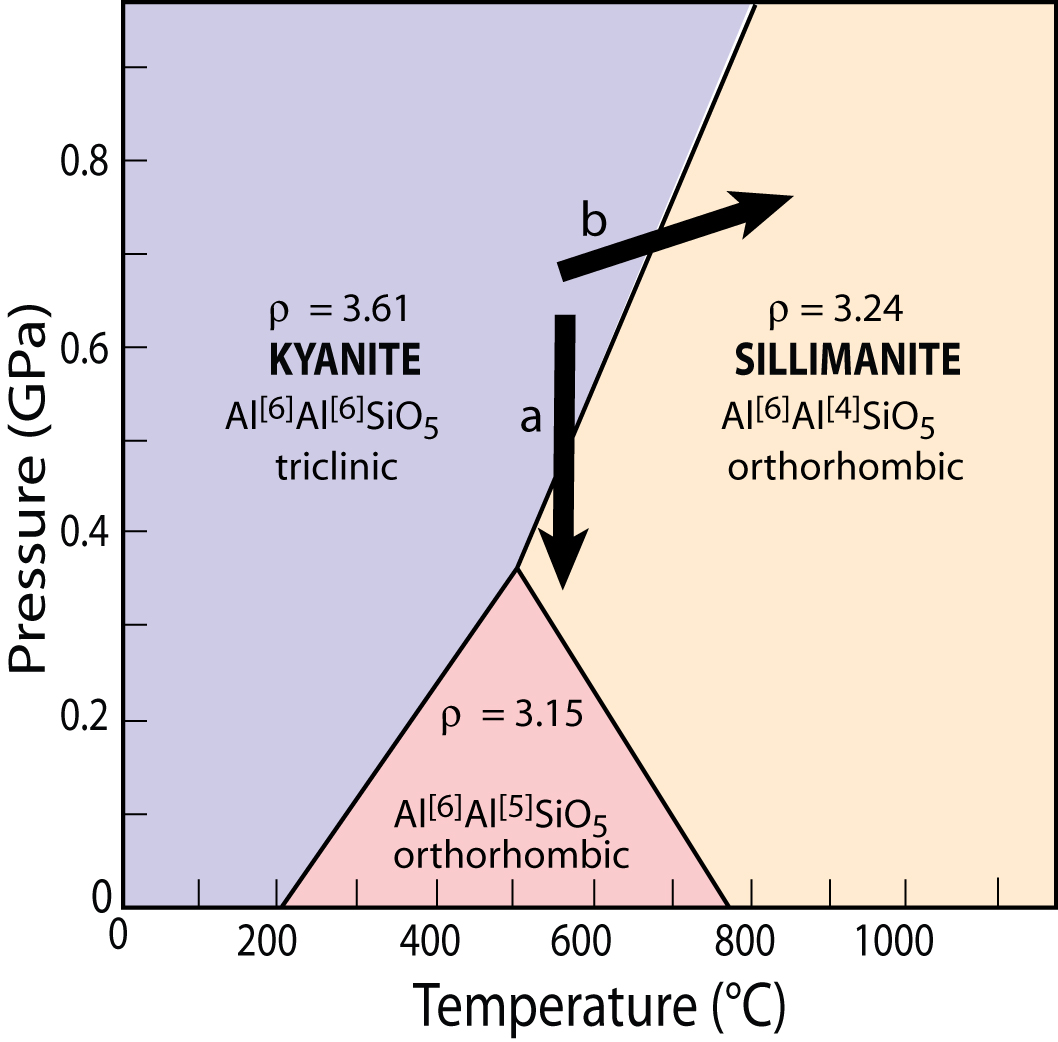
Phase Diagram from http://serc.carleton.edu/NAGTWorkshops/mineralogy/mineral_physics.html#what
|
Graphite and diamond are a well
known example of what differences in pressure
and temperature can do. They are also
polymorphs which are only made up of carbon.
They are the same chemically but physically
they are very different. On the Mohs hardness
scale where 1 is softest and 10 is hardest,
diamond is a 10 while graphite is a 1 or a 2.
This is a huge difference. Graphite is harder
than ones fingernail while a diamond is the
hardest known mineral. At lower pressures
graphite is the stable solid while diamond is
only stable of a pressure of about 10^4 atm.
Once the solid carbon is in the form of
diamond it rarely ever will turn back to
graphite. This is due to all the carbon bonds
in the diamond structure. There is not enough
energy for it to turn back. When this occurs
is called a metastable mineral. Below is the
specific pressures and temperatures for which
diamond and graphite exist.
|
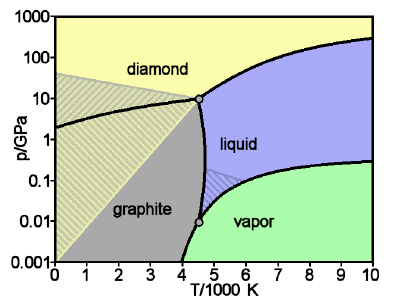
Phase diagram from http://www.chem1.com/acad/webtext/states/changes.html
|
|