Colors in the Aurora:
why and when which colors appear
When the particles in the plasma emit their photon of light, that photon contains certain properties that cause that light to be a certain color. The way these properties interact with one another is what induces the differences in how we perceive the color of the light.
The type of particle and the level of its excitement: Whether or not the particle is a proton or electron greatly influence what color we see. The level of excitement refers to the amount of
 energy it has stored before the photon
is emitted, not how eager it is to make light. When an electron has enough energy stored, it
sometimes becomes strong enough to split the air molecules of the upper atmosphere into oxygen
and
nitrogen
atoms. This is why the aurora typically
appears to be the signature colors that are emitted by these two atoms.
For example, oxygen usually emits red and green light. Green is the
most common aurora borealis color- it is also the most visible. We
don't see the red too much, because it is a redish brown that is at the extreme of
the visual light spectrum. Some of the visible colors are also mixtures
energy it has stored before the photon
is emitted, not how eager it is to make light. When an electron has enough energy stored, it
sometimes becomes strong enough to split the air molecules of the upper atmosphere into oxygen
and
nitrogen
atoms. This is why the aurora typically
appears to be the signature colors that are emitted by these two atoms.
For example, oxygen usually emits red and green light. Green is the
most common aurora borealis color- it is also the most visible. We
don't see the red too much, because it is a redish brown that is at the extreme of
the visual light spectrum. Some of the visible colors are also mixtures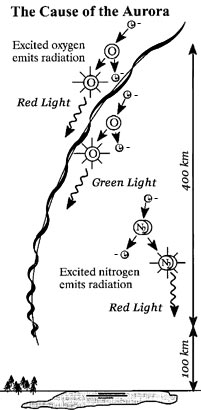 of colors from all of the emissions.
Purple is an example of this phenomenon, as it is a mixture of the red
and blue emissions from the nitrogen atoms.
of colors from all of the emissions.
Purple is an example of this phenomenon, as it is a mixture of the red
and blue emissions from the nitrogen atoms.Diagram courtesy of http://odin.gi.alaska.edu/FAQ/#color
The altitude of the excited particles also effects which colors are produced. This is because certain molecules are found in certain areas of the atmosphere. For instance, atomic oxygen (O as opposed to O2) is more common in higher altitudes, so the red aurora lights are typically above the other colors.
Courtesy of http://www.exploratorium.edu
This brings us to a HUGE realization: these lights are usually being emitted no less than 60 miles above the Earth's surface. They are so far away, our perception of them is skewed: the lights look immensely huge even from Earth, but we know that things look smaller if they are far away. So close up, they are actually MUCH larger and moving MUCH faster. This is even more mind-blowing because the higher auroras are seen at 200 miles above the Earth's surface!

Photo
courtesy
of
PBS.org