Yet another method of cooling objects, magnetic cooling, exploits the relationship between the effects of the magnetic field strength of an applied field and the entropy of an object.
One particular method of
magnetic cooling is Adiabatic
Demagnetization, which capitalizes on the paramagnetic
properties of some materials to cool those materials (usually in
gaseous form) down into the millikelvin -- or colder -- range.
This method can also be used to cool solid objects, but the
most drastic cooling in the fractions of a kelvin range are generally
accomplished for low-density gases that have already been greatly
cooled (around 3-4 K)
What
are Paramagnetic Materials?When an outside magnetic
field is applied to these materials, a
magnetic field that is parallel to the applied field is induced.
The
amount of particles that align with the
applied field depends on the field strength: if there is a higher field
strength, more
particles are aligned with the field.
The Process of Adiabatic
Demagnetization
- First, the sample to be cooled (typically a gas) is allowed
to touch a cold reservoir (which has a
constant temperature of around 3-4 K, and is often liquid Helium), and
a magnetic field is induced in the region of the sample.
- Once the sample is in thermal equilibrium with the cold
reservoir, the
magnetic field strength is increased. This causes the entropy
of the sample to decrease, because the system becomes more ordered as
the partilces align with the magnetic field. The temperature
of the sample is still the same as that of the cold reservoir at this
point.
- Then the sample is isolated from the cold reservoir, and
the magnetic
field strength is reduced. The entropy of the sample remains
the same, but its temperature drops in reaction to the reduction in the
magnetic field strength. If the sample was already at a
fairly low temperature, this temperature decrease can be ten-fold or
greater.
This process can be repeated, permitting the sample to be cooled to very low temperatures.
Limitations of the Process of Adiabatic Demagnetization
- If the sample material is an electronic
paramagnet, the
lowest
temperatures that can be attained using these methods are on the order
of 1 millikelvin.
What are Electronic Paramagnets? These are materials with net electronic magnetic moments. These materials are paramagnetic because electronic magnetic moments tend to align themselves with a magnetic field.
- If the sample is a nuclear
paramagnet, the lowest achievable
temperatures are
much lower (because the interactions between the dipoles are much
weaker). Nuclear paramagnets have been cooled down to 250
picokelvins, and it may be possible to cool them further.
What are Nuclear Paramagnets?
Materials with a net magnetic moment that is caused by the individual magnetic moments of the material's nuclei.
Nulear magnetic moments are about 1000 times smaller than electronic magnetic moments, so the dipole interactions are weaker. As a result, nuclear paramagnetic materials can be cooled to temperatures that are orders of magnitude less electronic paramagnetic materials can be cooled to.
The reason for this is that the interactions between the individual particles tend to create their own internal magnetic field when the applied magnetic field is very weak or absent. So, there is a limit to how weak the applied magnetic field can be, because at some point, the particle interactions cancel any further cooling effects of demagnetization. Since the dipole interactions between particles in nuclear paramagnetic materials are far weaker than those in electronic paramagnetic materials, the temperature and field strength limits are far lower.
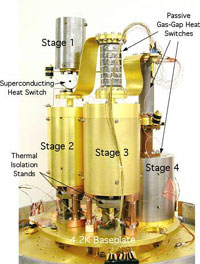
An adiabatic demagnetization cooler at NASA.
<http://constellation-x.nasa.gov/mission/technology/xms.html>
| Home | Vapor-Compression Refrigeration | The Peltier Effect and Thermoelectric Cooling | Laser Cooling and Optical Molasses | Magnetic Cooling | References |
| S H Price 26 March 2007 Physics 212 Web Project |