http://en.wikipedia.org/wiki/Image:System-boundary.jpg
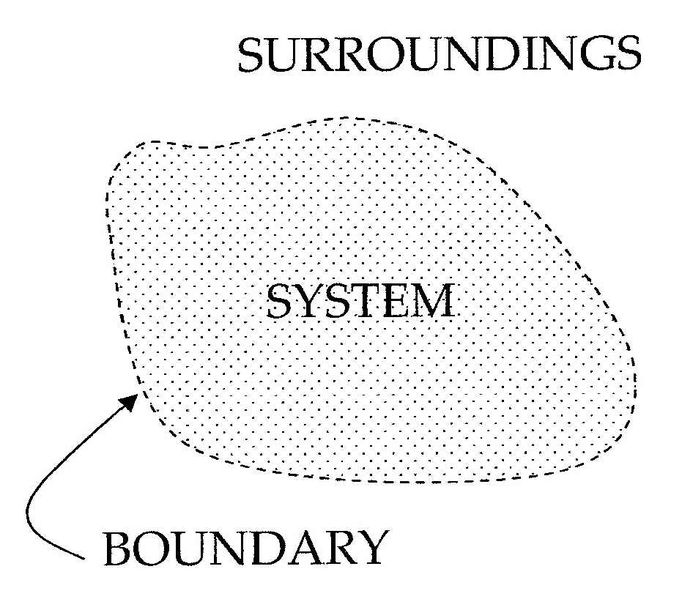
An important concept in thermodynamics is the system. A system is the region being studied. A system is separated from the remainder of the universe by a boundary which may be imaginary or not, but which limits the volume of region being studied. The possible exchanges of work, heat, or matter between the system and the surroundings take place across this boundary. There are five dominant classes of systems:
Isolated Systems – matter and energy may not cross the boundary.
Adiabatic Systems – heat may not cross the boundary.
Diathermic Systems - heat may cross boundary.
Closed Systems – matter may not cross the boundary.
Open Systems – heat, work, and matter may cross the boundary.
(book reference)

http://en.wikipedia.org/wiki/Image:System-boundary.jpg