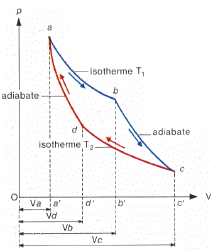
The Carnot cycle is the most efficient cycle possible. It consists of four basic reversible processes meaning that the cycle as a whole is also reversible. The four reversible processes are:

1. Reversible adiabatic compression of the gas.
During this step, the surroundings do work on the gas, compressing it and causing the temperature to rise from T2 to T1.
2. Reversible isothermal expansion of the gas at the "hot" temperature, T1.
During the step the expanding gas causes the piston to do work on the surroundings. The gas expansion is propelled by absorption of heat from the high temperature reservoir.
3. Reversible adiabatic expansion of the gas from T2 to T1.
During this process the gas continues to expand, doing work on the surroundings. The gas expansion causes it to cool to the “cold” temperature, T2.
4. Reversible isothermal compression of the gas at the "cold" temperature, T2.
during this process the surroundings do work on the gas, causing heat to flow out of the gas to the low temperature reservoir.
This process will continuously repeat itself following the Carnot Cycle.