Most materials fall into two categories: conductors and insulators. These materials conduct or do not conduct electricity, respectively. Now, how well this electricity can flow through a conductor is a measure known as resistance. Electricity flows by raising electrons from a lower energy level to a higher energy level in the atoms of the material.
Electron Bands. Britney's Guide - 2005
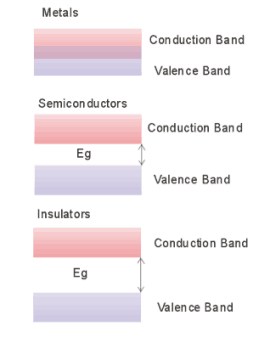
Semiconductors are insulators at very low temperatures, but at a suitable temperature, the additional thermal energy allows electrons to jump into the conduction band.
In atoms, electrons are divided into energy levels which tend to form bands. The highest, filled energy level at which electrons occupy is called the valence band and the first level above the valence band is known as the conduction band. The valence electrons do not participate in conduction. In metals, the valence and conduction bands overlap and allow free electrons to participate in conduction, however, insulators have an energy gap that is far greater than the thermal energy of the electron. Semiconductors are somewhere between these two extremes, having an energy gap around 1 eV. (Britney's Guide)
Semiconductors can be elements such as Germanium, Zinc, and Silicon, or compounds. Also, some semiconductor materials can be intrinsic (pure semiconductors) or extrinsic. An intrinsic semiconductor is normally used in conjunction with thermal or optical excitement to raise electrons from the valence band to the conduction band.


Intrinsic N-type P-type
Britney's Guide - 2005
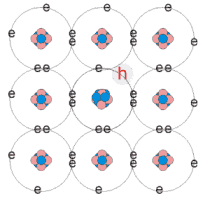
An extrinsic semiconductor is formed by "doping" the intrinsic semiconductor material with a VERY small number of impurity atoms. Doping takes an intrinsic semiconductor and adds or subtracts electrons from the material creating or destroying "holes". These holes flow in the opposite direction of the charge and are the result of an electron leaving it's parent atom and creating a vacancy for another electron to fill. The number of electrons headed in one direction is equal to the number of holes traveling in the opposite. Doping which creates holes is called p-type and doping which adds electrons to the semiconductor is called n-type. (Britney's Guide)
The most common n-type dopants for silicon are phosphorus and arsenic, while the most common p-type dopant for silicon is boron. When these two doping techniques are combined, a p-n junction is created. This junction is the basis of an electronic device called a diode.
The production of semiconductors requires a high degree of perfection in not only chemical purity but in crystalline perfection. The crystalline structures of semiconductors allow them to carry charge efficiently. However, if there are flaws in the structure, then energy bands can form inside of the lattice, interfering with the electrical operation of the semiconductor.
| Home | History | How They Work | Uses | Works Cited |