THE DISPLACEMENT ENGINE:
 Figure
1
Figure
1
THE DISPLACEMENT ENGINE:
 Figure
1
Figure
1
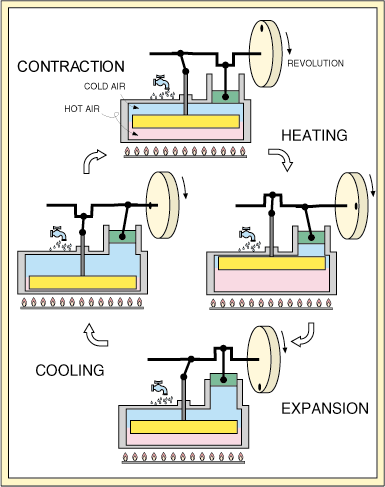
In the displacement engine there are two pistons.
The smaller piston shown in the illustration is the power piston.
All of the power for this model is provided by the power piston.
The second larger piston is the displacer piston. It's function is
to move the air between the hot and the cold sides of the air compartment.
It provides no power at all. The power piston for this model should
be 90 degrees out of phase from the displacer piston. This model
has four simple steps. Begining at the top of the illustration, the
first step is heating. The heating is caused by the movement of the
displacer piston so that most of the gas is on the hot side. The
temperature of the gas subsequently increases, causing an increase in pressure.
Because of this increase in pressure there is an expansion of the gas causing
the power piston to rise. Then, due to the 90 degree phase shift
between the two pistons, the displacer piston is moved, resulting in the
cooling of the gas. But when the gas is cooled, the pressure decreases,
causing a contraction in the gas, thereby pulling the power piston back
down. Then once again, due to the 90 degree phase shift, the displacer
piston follows causing the gas to shift to the hot side of the chamber.
The temperature of the gas then increases, and we are back to our first
step. This is the most basic model of the stirling engine.
THE TWO CYLINDER ENGINE:
 Figure 2
Figure 2
The two piston model is slightly more complex.
There are still two pistons, and they are still 90 degrees out of phase
from one another. However, in this model power is supplied by both
pistons, and the displacement of the gas is caused by both as well.
Yet it does follow the same basic process. Once agian, starting from
the top of the illustration, the first step is the heating of the gas in
the chamber. The flywheel is turning, and thus the cold piston moves
up, and the hot piston moves down. This causes the gas to flow to
the hot side. This then causes an increase in the temperature of
the gas. The gas therefore expands, pushing both pistons downward.
At this point the inertia of the flywheel causes it to continue rotating
which in turn raises the hot piston and pulls the cold piston downward.
The gas is then pulled to the cold side of the chamber, and the temperature
of the gas is decreased. But this decrease in temperature causes
the gas to contract, and therefore pulls both pistons upwards. Then,
once again, the inertia of the flywheel pulls the hot piston down and pushes
the cold piston up. Thus the gas flows to the hot side of the chamber
and is heated, leaving us where we began.