How microwaves heat food
An interesting
effect of microwave ovens is their ability to heat some
materials very easily, while other materials are
effected very little or not at all.
Microwave ovens primarily use the food's water content to heat the system. This has to do with the molecular structure of water and the frequency of electromagnetic radiation used in microwave ovens.
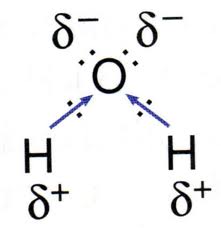
Water is a polar molecule, or a dipole. This means that the structure of water causes one side of the molecule to be positively charged while the other has a relative negative charge.
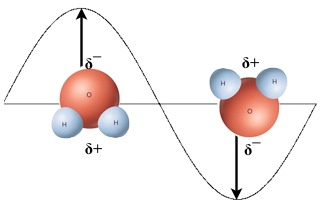
When microwaves are absorbed by the food in the cooking chamber, they interact with the net polar compounds in the food. Each time a microwave interacts with a water molecule, it 'flips' the orientation of the molecule when the oppositely charged part of the wave interacts with the polar molecule. Each time a water molecule is flipped, a tiny amount of electromagnetic energy is converted into thermal energy through friction.
Materials such as glass are not as polar on an atomic level, which is why they will not heat up when microwaved.
Other molecules, such as fats and sugars, are heated much less efficiently than water on account of their relatively small dipole moment.
Microwave ovens primarily use the food's water content to heat the system. This has to do with the molecular structure of water and the frequency of electromagnetic radiation used in microwave ovens.
Water is a polar molecule, or a dipole. This means that the structure of water causes one side of the molecule to be positively charged while the other has a relative negative charge.
When microwaves are absorbed by the food in the cooking chamber, they interact with the net polar compounds in the food. Each time a microwave interacts with a water molecule, it 'flips' the orientation of the molecule when the oppositely charged part of the wave interacts with the polar molecule. Each time a water molecule is flipped, a tiny amount of electromagnetic energy is converted into thermal energy through friction.
Materials such as glass are not as polar on an atomic level, which is why they will not heat up when microwaved.
Other molecules, such as fats and sugars, are heated much less efficiently than water on account of their relatively small dipole moment.