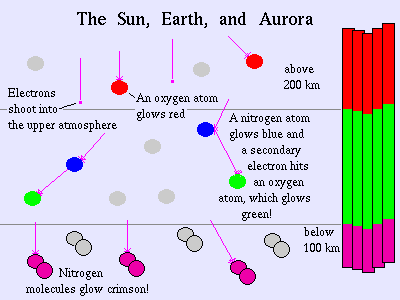
The Aurora Borealis is most often seen in a striking green color, but it also occasionally shows off its many colors ranging from red to pink, blue to purple, dark to light. The reason that the aurora is seen in so many colors is that our atmosphere is made up of many different compounds like Oxygen and Nitrogen. When the charged particles that come from the sun hit the atoms and molecules of the Earth's atmosphere, they excite those atoms, giving off light. Different atoms give off different colors of the spectrum when they are excited. A familiar example is the Neon lights that we see on many business signs in our modern world. The Neon lights contain the gas Neon. These lights have electricity run through them to excite the Neon gas. When the Neon is excited, it gives off a brilliant red-orange color. The Neon lights are the same idea as the aurora, only on a lot smaller scale.


| Aurora Home | How Auroras Formed | Aurora Colors | Where They can be Seen | Aurora on Jupiter | Aurora in Folklore | Bibliography |