Shape theory makes sense
intuitively and has been the historic model for olfaction, but
current research points to a slightly more complex model.
Vastly different-shaped molecules sometimes have the same
odor, while very similarly shaped compounds sometimes smell
quite different from each other. Shape theory cannot explain
these differences: by the theory, if two molecules have the
same shape, the must have similar smells; likewise if they
have different structures, they should also have different
smells. But some animals can distinguish between different
isotopes of the same molecule (1). In these cases, the
molecular composition and structure are the same, but
different isotopes have slightly different masses.
Shape theory makes sense
intuitively and has been the historic model for olfaction, but
current research points to a slightly more complex model.
Vastly different-shaped molecules sometimes have the same
odor, while very similarly shaped compounds sometimes smell
quite different from each other. Shape theory cannot explain
these differences: by the theory, if two molecules have the
same shape, the must have similar smells; likewise if they
have different structures, they should also have different
smells. But some animals can distinguish between different
isotopes of the same molecule (1). In these cases, the
molecular composition and structure are the same, but
different isotopes have slightly different masses.
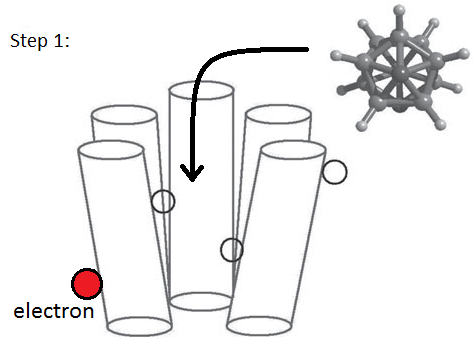
The answer to these problems may lie in the
molecule's vibrational patterns. In place of shape theory's
'lock and key' analogy, the vibration theory proposes a 'swipe
card' method: as the molecule vibrates olfactory receptors,
electrons will jump between different areas in the receptor by
means of quantum tunneling (1). If two molecules had very
different shapes but the same vibrational patterns, we would
expect them to have similar smells. Likewise if two molecules
have similar structures but different vibrational patterns,
such as the changed mass of isotopes, then the two molecules
probably have different odors.This theory is still
in the developmental stages, but it seems to hold water and
appears to be a more accurate mechanism for olfaction.
Nancy Rica Schiff
 The
Physics of Smell
The
Physics of Smell The
Physics of Smell
The
Physics of Smell makes intuitive sense to you, it may be
a good idea to brush up on electron tunneling
before reading this article...
makes intuitive sense to you, it may be
a good idea to brush up on electron tunneling
before reading this article... Shape theory makes sense
intuitively and has been the historic model for olfaction, but
current research points to a slightly more complex model.
Vastly different-shaped molecules sometimes have the same
odor, while very similarly shaped compounds sometimes smell
quite different from each other. Shape theory cannot explain
these differences: by the theory, if two molecules have the
same shape, the must have similar smells; likewise if they
have different structures, they should also have different
smells. But some animals can distinguish between different
isotopes of the same molecule (1). In these cases, the
molecular composition and structure are the same, but
different isotopes have slightly different masses.
Shape theory makes sense
intuitively and has been the historic model for olfaction, but
current research points to a slightly more complex model.
Vastly different-shaped molecules sometimes have the same
odor, while very similarly shaped compounds sometimes smell
quite different from each other. Shape theory cannot explain
these differences: by the theory, if two molecules have the
same shape, the must have similar smells; likewise if they
have different structures, they should also have different
smells. But some animals can distinguish between different
isotopes of the same molecule (1). In these cases, the
molecular composition and structure are the same, but
different isotopes have slightly different masses.