Thermodynamics
Just like everything else, marine mammals are affected by the Laws of Thermodynamics
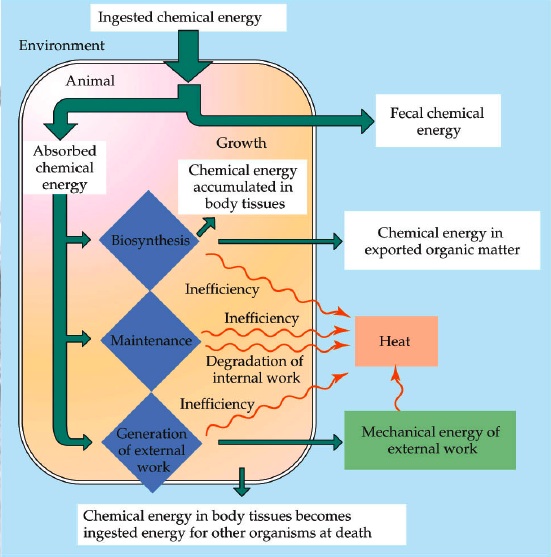
The 1st Law of Thermodynamics states that energy is conserved (it cannot be created nor destroyed, only transformed), and that the change in internal energy of a closed system is equal to the heat added to it by heating, minus the work done by the system on its environment (Giancoli 409).
This means that for a marine mammal to keep its body temperature constant (which is what it will try to do, because marine mammals are homeothermic endotherms), it must make the amount of heat it receives equal to the amount of heat it puts out. (Horstmann-Dehn, 2012)
The 2nd Law of Thermodynamics states that heat flows spontaneously from hot places to cold ones, and that in any natural process, some energy will become unavailable to do work (it will be lost as heat). (Giancoli 409) This is also relevant to marine mammals because it means that they are constantly losing heat to the environment (because water temperature in the ocean is always lower than the marine mammals' body temperature), and also because it means that in every metabolic process in their body, they lose some energy as heat & can no longer use it. (Horstmann-Dehn, 2012)