Where
is
the rate of heat transfer, A is the surface area,
is
the absolute temperature of the surface, and
is the
Stefan-Boltzmann constant ().
This equation gives an idealized maximum rate of radiation.
Ideal surfaces that emit radiation at this maximum rate are
called blackbodies, and the radiation that blackbodies emit is
called blackbody radiation. The actual radiation emitted by
surfaces is less than their blackbody radiation at the same
temperature, and is given by:
(W)
Where
is
the emissivity of the surface. Emissivity measures how well
a surface approximates a blackbody, and ranges in value
between 0 and 1, where a value of 1 is a blackbody.
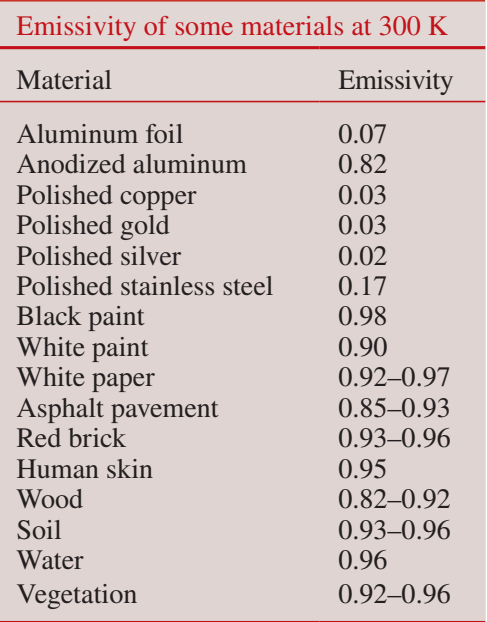
Table of some emissivities of some materials (Çengel 2010)
Table of some emissivities of some materials (Çengel 2010)
Emissivity isn't the only important
radiation related property of a surface. Another important
property is absorption, which gives the fraction of
radiation energy that intercepts a surface that is absorbed
by the surface. Absorptivity () has
a value between 0 and 1, where a value of 1 is a perfect
absorber and a blackbody. So, a black body is a perfect
emitter with a emissivity of 1, and a perfect absorber with
an absorptivity of 1.
Emissivity and absorptivity are related by Kirchhoff's Law, which states that for a body in thermodynamic equilibrium, the emissivity is equal to the absorptivity.
The other ways that electromagnetic radiation can interact with a material is through reflection and transmission. Reflection occurs when radiation bounces off of a surface and is redirected, and transmission occurs when radiation passes through a target. Radiation from a source can be reflected, absorbed, or transmitted when it intercepts a surface, and the energy related to these is related with:
It can be difficult to determine what the rate of heat transfer is between two objects because of all of the variables that are involved, such as the properties of the surfaces of the objects, the object's orientation relative to each other, and the properties of the medium that radiation passes through. However, the math to solve the rate of heat transfer between two objects can be simplified if: the emissivity and surface area are relatively low at a temperature and is completely enclosed by a much larger surface at and the medium through which the radiation passes through has a negligible effect on the radiation, which will lead to the equation:
Emissivity and absorptivity are related by Kirchhoff's Law, which states that for a body in thermodynamic equilibrium, the emissivity is equal to the absorptivity.
The other ways that electromagnetic radiation can interact with a material is through reflection and transmission. Reflection occurs when radiation bounces off of a surface and is redirected, and transmission occurs when radiation passes through a target. Radiation from a source can be reflected, absorbed, or transmitted when it intercepts a surface, and the energy related to these is related with:
It can be difficult to determine what the rate of heat transfer is between two objects because of all of the variables that are involved, such as the properties of the surfaces of the objects, the object's orientation relative to each other, and the properties of the medium that radiation passes through. However, the math to solve the rate of heat transfer between two objects can be simplified if: the emissivity and surface area are relatively low at a temperature and is completely enclosed by a much larger surface at and the medium through which the radiation passes through has a negligible effect on the radiation, which will lead to the equation:
(W)
Back to home
page