Image 1: Method of heat transfer through
through a fluid. Higher energy
particles transfer energy though collisions with lower energy
electrons. Image accessed from: https://courses.lumenlearning.com/boundless-physics/chapter/methods-of-heat-transfer/
The rate of conduction through a substance is
controlled by:
k is dependent on the material that heat transfer is passing through. Some common k values are:

Back to Home page
where
is the rate of heat transfer (SI units in watts), k is the
conductivity of the substance, A is the cross-sectional
area of the substance normal to the direction of heat
transfer, Δ T is the temperature
difference across the substance, and Δ x is the thickness of the
substance. In in differential form, the equation becomes
Fourier's law of heat conduction:
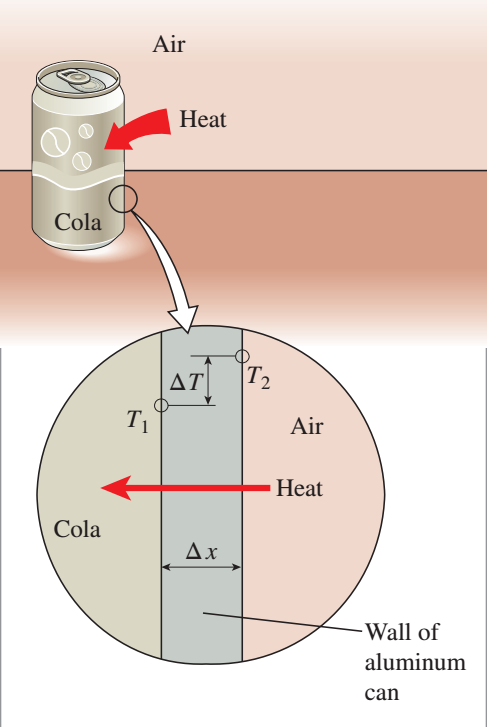
Image 2: visualization of the variables that
control the rate of conduction through a substance.
Image accessed from: Çengel, Y. A., Boles, M. A., & Kanoğlu, M. (2020).
Thermodynamics an engineering approach [9th edition]. Singapore: McGraw-Hill.
Image accessed from: Çengel, Y. A., Boles, M. A., & Kanoğlu, M. (2020).
Thermodynamics an engineering approach [9th edition]. Singapore: McGraw-Hill.
k is dependent on the material that heat transfer is passing through. Some common k values are:
Image 3: thermal conductivity of materials.
Image accessed from: Çengel, Y. A., Boles, M. A., & Kanoğlu, M. (2020).
Thermodynamics an engineering approach [9th edition]. Singapore: McGraw-Hill.
Image accessed from: Çengel, Y. A., Boles, M. A., & Kanoğlu, M. (2020).
Thermodynamics an engineering approach [9th edition]. Singapore: McGraw-Hill.
Back to Home page