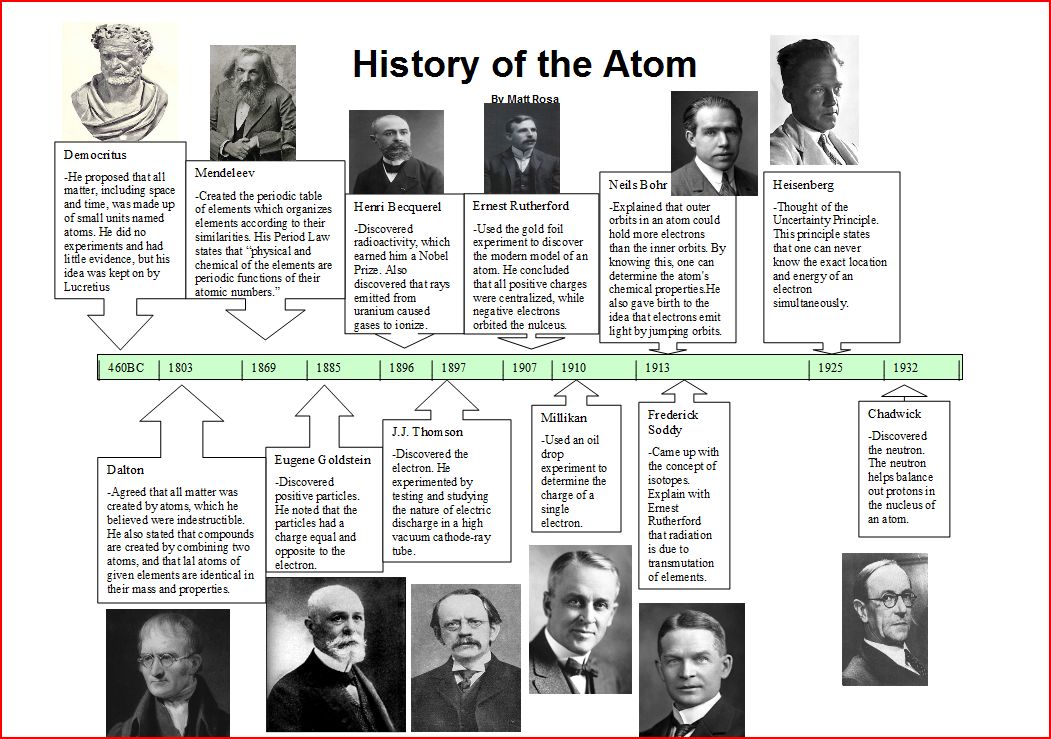
 500-400 BC- Multiple philosophers such as Democritus and Lucretius theorized about atoms. They thought that all objects where were made up of small particles but due to their time, they could not provide proof of their theory. 1800's- John Dalton coined the atomic theory where he stated that all matter is made up of atoms and that atoms make up elements and compounds. He believed atoms could not be split but that was later disproved. Closer to the center of the century, Dimitri Mendeleev created the periodic table and discovered an impressive number of elements despite a lack of technology. Later on, Eugene Goldstein discovered protons using discharge tubes and magnetic fields and hinted the presence of a negatively charged particle. Becquerel went on to discover radioactivity through the observation of an element called uranium. To conclude the century for atomic discoveries, J.J. Thomson observed an experiment using cathode rays in a vacuum to discover the presence of electrons. 1900's- Ernest Rutherford began the 20th century by discovering that atoms are composed of a very dense nucleus with lots of empty space through the gold foil experiment. He shot particles through a very fine sheet of gold and discovered that many of the particles went straight through while some of the others were deflected which meant that there must have been space between the atoms. Robert Millikan continued J.J. Thomson's discovery of the electron by using an oil drop experiment to determine the charge of a single electron at an impressive -0.0000000000000000001602 coulombs. Further electron discovery was done by Neils Bohr where he discovered that electrons are found in orbits through the excitation of electrons and the color of light emitted. Frederick Soddy went on to discover isotopes, meaning the same elements just with varying neutron counts (though neutrons were not known knowledge at the time) and realized that radioactivity was due to the deterioration of an unstable atom. Heisenberg discovered that it is impossible to locate an electrons exact position and to know its energy at the same time. James Chadwick concluded the major discoveries of the 1900's by discovering the presence of the neutron in the nucleus of the atom which was a fundamental concept in the understanding of future quantum mechanics. Overview
|
|
|
