Background
Thomas Seebeck discovered that the heating of coupled dissimilar metals, a thermocouple, produced a voltage across their cool ends. He also found that in connecting the cool ends a circuit was made that would sustain an electric current that was proportional to the heat gradient maintained across the circuit.

Seebeck's discovery was made only a few decades after Alessandro Volta conceived of the first chemical battery in 1800. Early understanding of battery function was imperfect, so the first chemical batteries were unable to produce a steady voltage. The work of Joseph Fourier (of Fourier transforms) and Leopoldo Nobili on Seebeck's observations contributed to the first stable power sources (example pictured above). It was these combinations of thermocouples, the thermopile, that allowed George Ohm to complete his research on voltage and current.
Limited by Efficiency
By 1840 Batteries became far more reliable and safe, mechanical power generation had been discovered, and thermoelectric power generation became something of a novelty with only very specific uses.The simple limitation was and still is inefficiency. Native metallic conductors are only capable of delivering 5-8% efficiency. That said, it is possible to increase the power output of a device by increasing its size and number of elements. One thermopile from the end of the 19th century comprised of 3000 thermocouples was capable of delivering 192 Watts at 3.5 Amps. It achieved this with 20 lbs of coal/hour: .2% efficiency. Modern units are capable of producing many kilowatts.
Recently, thermoelectric generation has enjoyed renewed attention with a discovery made by Abram Ioffe in 1929- semi-conductors are much better thermoelectric performers.

As the temperature of a metallic conductor is increased, its conductivity decreases: not ideal for creating current from heat. Semi-conductors behave in the opposite way where conductivity increases with temperature. Semi-conductors are either pure elements like silicon or composites like lead telluride (pictured above in its mineral form, altaite).
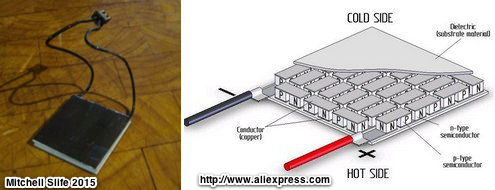
By their nature semi-conductors are not of themselves useful for conducting current, it is therefore necessary to alter their structure- a process called doping. Semi-conductors can be doped with materials that want to give up electrons, an N type semi-conductor, or they can be doped with materials that want electrons, a P type semi-conductor. When coupled together and exposed to a heat gradient we get the same thermoelectric effect- but with much greater efficiencies. Research in this field continues, and as of 2012 Northwest University has made advances manipulating lead telluride to achieve up to 20% efficiency.
How efficient thermoelectric generators may become is considered with the Peltier effect.