After falling down the rabbit hole, Alice finds herself in a room with a very small door. In order to fit through the door she must drink a vial that makes her shrink.

Luckily when Alice shrunk she kept her mouth open to let the air out of her lungs. Had she not done this, the air in her lungs would have been compressed and probably burst through her chest. Lets calculate how much pressure would have been built up in her lungs had she kept her mouth closed!
To calculate the pressure we will use the ideal gas law PV = nRT. Since no air will be leaving her body the moles of gas will remain constant and we will assume the temperature does as well. By manipulating the equation I came up with the following.
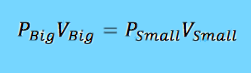
The volume of a normal human's lungs is roughly 6 L. By reviewing the film I determined she shrunk by a factor of 0.063. Therefore we will use 0.38 L as the final volume of her lungs. 1 atm will be used as the initial pressure since her lungs would be equalized with standard atmospheric pressure.
By plugging the values into the equation I determined that the pressure in her lungs would be 15.75 atm. That is over 230 psi and almost twice the pressure of a standard propane tank.

